A Review of the Prevention and Treatment of Influenza
RELEASE DATE
July 1, 2025
EXPIRATION DATE
July 31, 2025
FACULTY
Katherine Hale, PharmD, BCPS, MFA
Freelance Medical Writer
Tri-Cities, Washington
FACULTY DISCLOSURE STATEMENTS
Dr. Hale has no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-25-052-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities
GOAL
To update pharmacists on the prevention and management of influenza.
OBJECTIVES
After completing this activity, the participant should be able to:
- Discuss the incidence and burden of influenza.
- List age-appropriate vaccines for influenza prevention and their manufacturing methods.
- Identify appropriate antiviral interventions for patients with known or suspected influenza infection or for postexposure chemoprophylaxis.
- Describe the role of the pharmacist in influenza prevention and treatment.
ABSTRACT: Influenza infection significantly burdens healthcare systems and those infected each year, with estimates of up to 81 million illnesses and more than 100,000 deaths in 2024- 2025. Direct and indirect costs may reach $11 billion or more, and illness frequently affects school or work productivity. Many vaccine options are available for persons of all ages and health status. Neuraminidase inhibitors and a newer cap-endonuclease inhibitor are available for influenza treatment and chemoprophylaxis. Pharmacists may play a role in continued efforts to address vaccination barriers and potential antiviral and vaccine shortages and improve methods for prevention and treatment of influenza.
Influenza is a single-stranded helical ribonucleic acid (RNA) virus that typically circulates from late fall to early spring in the United States, significantly burdening the healthcare system and those infected.1,2 Preliminary estimates for the 2024-2025 influenza season indicate that 47 million to 81 million illnesses, 21 million to 37 million medical visits, 610,000 to 1.3 million hospitalizations, and 26,000 to 130,000 deaths have occurred due to influenza.3 Estimated averages of $8 billion ($4.8-$13.6 billion) in indirect and $3.2 billion ($1.5-$11.7 billion) in direct medical costs based on individuals who were ill but may not have sought medical care, days of lost productivity, outpatient office visits, emergency department visits, hospitalizations, and deaths have been attributed to influenza.4
A review of influenza burden from the 2010-2011 to 2023-2024 seasons indicated that 2017-2018 resulted in more symptomatic illness (40 million) and the highest number of hospitalizations (700,000; 95% uncertainty interval [UI] 550,000 to 1 million) and deaths (51,000; 95% UI 36,000 to 98,000). No estimates were available for the 2020-2021 season owing to minimal influenza activity. This has been attributed to the measures taken during the COVID- 19 pandemic to limit its spread.5 Seasons occurring from 2011-2012 and 2021-2022 had the lowest recorded hospitalizations and deaths.6
Productivity lost owing to missed days of school or work has been documented.7-10 Patients who are diagnosed with influenza have reported up to 45% more work hours missed (20.5 vs.15.0 missed hours, P <.001) and identified decreased productivity.9 Many employees report working while experiencing influenza or influenza-like symptoms (42% to 89%), and up to 74% of employees missed 1 or more days of work (0.5 to 5.3 days). Working caregivers were found to miss an average of 1 to 2 workdays to care for children or family members with influenza or influenza-like illness.8 Additionally, a study of 11 influenza seasons attributed 2,077 school closures to influenza or influenza-like illness, frequently citing increased absenteeism of students/staff due to illness (more than 50%).11 Multiple factors may increase influenza complications (TABLE 1). To reduce the burden of and complications from influenza infection routine, yearly vaccination is recommended by the CDC and the Advisory Committee on Immunization Practices (ACIP) for all age groups.1,2

INFLUENZA VACCINES
Influenza A is classsified by the surface antigens neuraminidase (NA) and hemagglutinin (HA) that determine the subtype (e.g., H1N1). Currently, 11 N and 18 H subtypes have been identified, with 8 H (H1-3, H5-7, H9, and H10) and 6 N (N1, N2, and N6-9) detected in humans. B/Yamagata and B/Victoria are known influenza B lineages.1
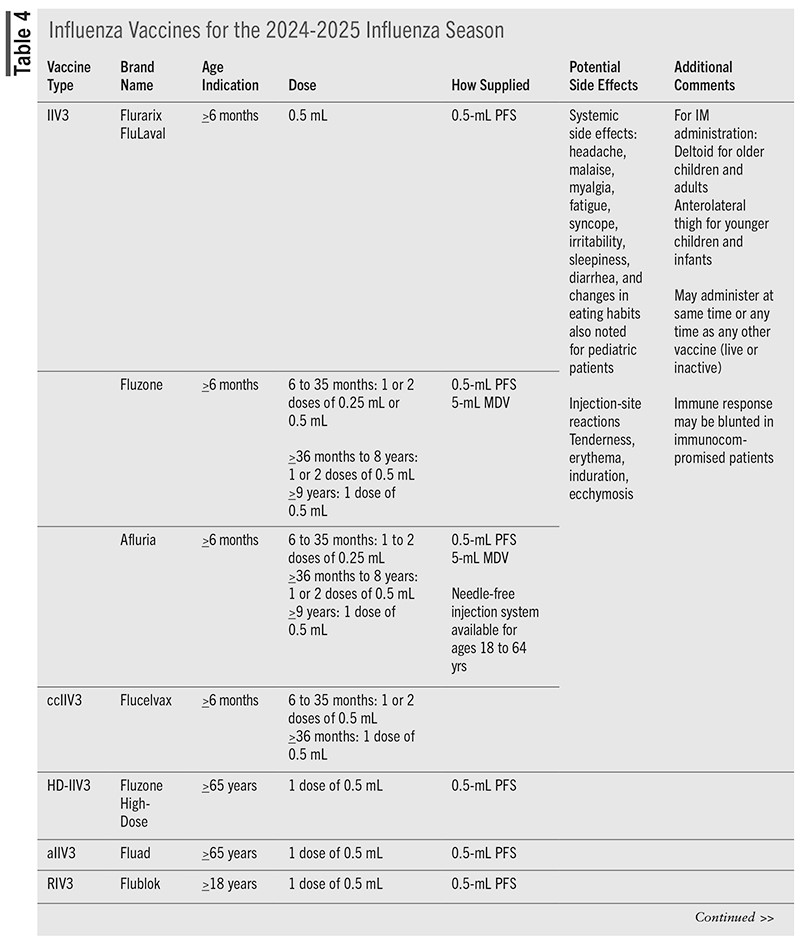
Vaccines are available as inactivated influenza vaccine (IIV), live attenuated influenza vaccine (LAIV), and recombinant influenza vaccine (RIV). IIV is further categorized as cell culture–based (ccIIV), high-dose (HD-IIV), MF59-adjuvanted (aIIV), and standard dose (SD-IIV). Trivalent vaccines contain two influenza A subtypes and one influenza B type. Quadrivalent vaccines contain one additional influenza B type.1,2
Methods of Vaccine Production
In 2023, the World Health Organization (WHO) reported that 88% of seasonal and pandemic influenza vaccine production capacity globally (1.53 billion doses seasonal vaccine) relied on embryonated eggs. About 11% was cell culture–based.12
Egg-based vaccine manufacturing requires seed virus injection into fertilized chicken eggs followed by growth and incubation over several weeks. Virus is harvested from egg whites, purified, and brought to a standardized vaccine potency. One egg produces one to two vaccine doses.13 Accordingly, five to 10 eggs may be required to produce 10 doses of vaccine. Therefore, billions of eggs are needed to produce enough vaccine worldwide. Egg-based vaccine manufacturing takes 8 to 10 months, but it is considered a year-long process. Using numbers from the previous influenza season, the quantity of eggs that are needed to produce the estimated required upcoming season's vaccine doses is estimated 6 months prior to injection of the seed virus into the embryonated egg.13
Cell culture–based vaccine was developed as an alternative to egg-based production. Its advantages include reduced production time, increased capacity, and reliability. Production begins with thawing cell-line "seed" lots, followed by preculture cell propagation in a nutrient medium. Influenza virus is introduced into the cell line upon reaching a prespecified cell density. Virus is harvested after 3 days. Cell lines that are used for vaccine production include Madin Darby canine kidney cells (MDCK), Vero cells (kidney epithelial cells from an African green monkey), and PER.C6 (human retina–derived cell line).14,15
RIV is produced using baculovirus expression vetor systems (BEVS).16 BEVS are recombinant baculoviruses containing a transcribed protein of interest.17 High yields of protein are obtained via replication of BEVS in cultured insect cells or larvae. Moths (lepidopteran insects) generate cell lines that are used for the BEVS.18 RIV in the U.S. is produced using the expresSF+ continuous cell line from the Sf9 cells of Spodoptera frugiperda, a fall armyworm, and the BEV Autographa californica nuclear polyhedrosis virus.19
Vaccine Hemagglutinin Content or Virus Count
Standard-dose egg-based, cell culture–based, and adjuvanted influenza vaccines contain 15 mcg HA of each virus per 0.5-mL dose (or 7.5-mcg/0.25-mL dose). Total HA content for trivalent IIV is 45 mcg per 0.5-mL dose. High-dose vaccine contains 60 mcg per virus included in the vaccine. LAIV contains 10 fluorescent focus units of each vaccine virus per 0.20mL dose (6.5-7.5 mcg). RIV contains 45 mcg HA per virus strain per dose, or 135 mcg/dose for trivalent vaccine.2
Vaccine Composition
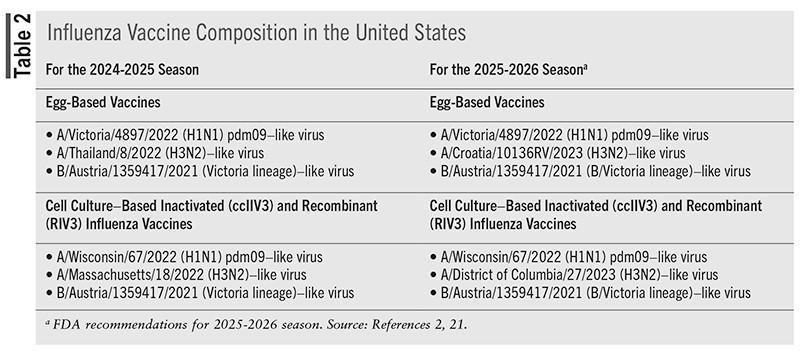
For the 2024-2025 influenza season, all vaccines were trivalent and contained two influenza A types and one B type from the B/Victoria lineage.2 Previously, quadrivalent vaccines containing a strain from the B/Yamagata lineage were recommended; however, there has been no confirmed detection of the B/Yamagata lineage since 2020. Therefore, the WHO determined that inclusion of the B/Yamagata lineage was not necessary.20
TABLE 2 identifies influenza vaccine composition for the 2024-2025 season and recommended composition for 2025-2026.
Vaccine Effectiveness
Common questions regarding influenza vaccination are why yearly vaccination is recommended and why illness may still occur despite vaccination. Antigenic drift, in conjunction with waning immunity, may be the culprit. Viral evolution may result in small changes in HA and NA that accumulate over time. The human immune system may not recognize new strains, resulting in increased influenza, potential for more than one episode of influenza in one season, and risk of an epidemic.1
Waning vaccine immunity during a single season has been evaluated.22-24 In a study examining seven influenza seasons (2010-2011 to 2016-2017), a 16% increase in the odds of testing positive for influenza occurred every 28 days between initial vaccination and influenza testing.22 Pooled analysis of six influenza seasons (2018-2019 to 2022-2023) found that the odds of testing positive for any influenza increased each week from initial vaccination (4.9%; 95% CI 2.0-8.0%).23 Additional studies identified that the odds of infection with A(H3N2) increased 1.12 times every 2 weeks.24
Vaccine Timing
Optimal vaccination timing is multifactorial, taking into consideration seasonal influenza variations, potential waning immunity, age, potential missed opportunities to vaccinate, pregnancy status, and potential for waning immunity.2
Most adults should be vaccinated in September and October. Administration in July and August, however, may be considered for children needing a two-dose series, children requiring only one vaccine dose (to prevent future missed vaccination opportunities), and women in the third trimester of pregnancy (to reduce the risk for influenza infection in infants aged <6 months whose age precludes vaccine administration). Booster doses are not recommended.2
High-Dose Influenza Vaccine
HD-IIV is indicated for adults aged >65 years and may be used for persons aged 18 to 64 years with a history of solid organ transplant.2 A systematic review and meta-analysis compared the relative vaccine effectiveness (rVE) of HD-IIV and SD-IIV in more than 22 million adults aged >65 years across 10 influenza seasons. The review found that protection was higher with HD-IIV (rVE, 15.9%, 95% CI 4.1%-26.3%) and more influenza-related hospitalizations were prevented (rVE, 11.7%, 95% CI 7.0%-16.1%).25 HD-IIV reduced all-cause hospitalization (rVE 7.3%; 95%, CI 4.5%-10.0%) and pneumonia/influenza hospitalizations (rVE, 23.5%, 95% CI 12.3%-33.2%) compared with SD-IIV in a meta-analysis of five trials (N = 105, 68%).26 Studies evaluating the use of HD-IIV in Medicare participants and veteran populations across different influenza seasons found HD-IIV more effective than SD-IIV in the prevention of influenza complications and hospitalizations.27-30
M59 Adjuvant
M59 is an oil-in-water emulsion of squalene oil combined with influenza antigen to create a stronger immune response. Combining M59 adjuvant with influenza antigen results in higher levels of HA-inhibiting antibodies and memory T and B cells, increasing vaccine immunogenicity.31 Studies have shown that aIIV is comparable to HD-IIV in reducing influenza illness, complications, and hospitalizations in adults aged >65 years.32,33
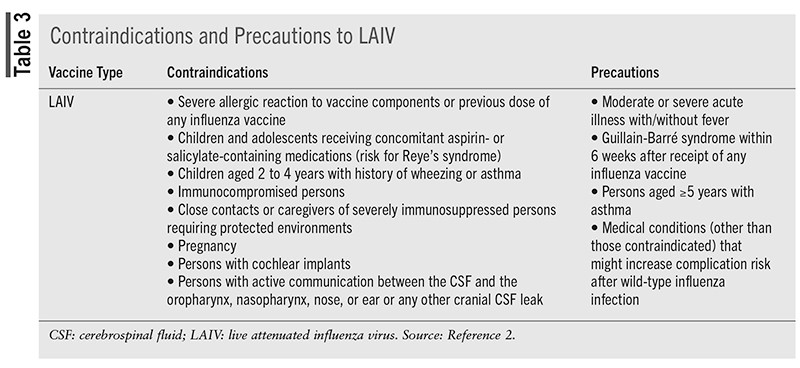
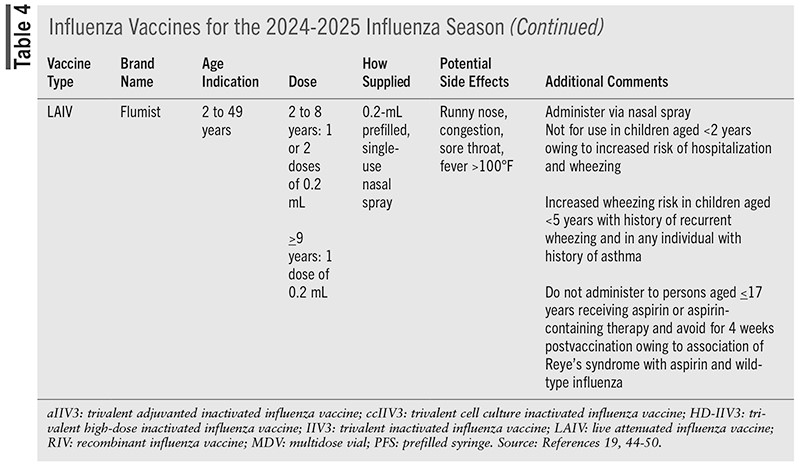
Live Attenuated Influenza Vaccine
LAIV is attenuated, temperature-sensitive, and cold-adapted.1,2,34,35 LAIV is administered via nasal spray and is indicated for individuals aged 2 to 49 years. For the 2025-2026 season, LAIV has been approved for self-administration at home by adults aged 18 to 49 years or by a parent or caregiver for those aged 2 to 17 years.35,36 Studies of at-home administration found that vaccine administration was successful, with few adverse effects, no duplications in influenza vaccination, and accurate updates of state immunization registries. Seroresponse and immunogenicity did not differ between at-home and in-clinic administration.37,38 Information regarding incorrect at-home administration of LAIV is limited.
For persons taking antiviral therapy for influenza treatment/prevention, LAIV administration is not recommended until 48 hours following antiviral cessation.34,39-42 Antiviral therapy should not be started until 14 days post-LAIV vaccination due to concern that antiviral therapies may inhibit replication of the live vaccine virus.34,39-42 Further recommendations from the ACIP, however, take into account the length of peramivir and balaxovir half-lives. Peramivir should be avoided 5 days before to 2 weeks after LAIV vaccination and baloxavir 17 days before to 2 weeks after vaccination.2
LAIV may be administered at any interval with inactivated vaccines. If not administered simultaneously, LAIV should be administered at least 4 weeks before or 4 weeks after other live vaccines.2 Contraindications and precautions to administration of LAIV are detailed in TABLE 3.
RECOMMENDATIONS REGARDING INFLUENZA VACCINES
Influenza vaccine recommendations are updated yearly by the ACIP.2 The ACIP guidelines for the 2024-2025 influenza season continued to recommend that all individuals aged 6 months or older with no vaccine contraindications receive an age-appropriate influenza vaccine. For 2024-2025, recommendations were updated to include HD-IIV3 and aIIV3 as acceptable options for solid organ transplant recipients aged 18 to 64 years who are also receiving immunosuppressive pharmacotherapy. RIV, aIIV, and HD- IIV are recommended for adults aged >65 years owing to increased risk of severe influenza-related complications for that age group. If RIV, aIIV, and HD-IIV are not available, however, SD-IIV should be administered.2 LAIV should be avoided in immunocompromised persons.2,34
Two doses of influenza vaccine, spaced 4 weeks apart, are recommended for children aged 6 months to 8 years with unknown vaccination status or who have never received influenza vaccine. Children aged 9 years or older should receive only one vaccine dose. If a child needs two doses but turns age 9 years between dose 1 and dose 2, the second dose should still be administered.2,43
A history of Guillain-Barré syndrome within 6 weeks of receipt of influenza vaccine is a precaution for any influenza vaccine, as is moderate or severe acute illness with or without fever.2 Contraindications to use of egg-based IIV include a history of severe allergic reaction, such as anaphylaxis, to any component of the vaccine or previous dose of any type of influenza vaccine. For ccIIV, a history of severe reaction to a previous dose or any component of ccIIV is a contraindication to use, while a history of severe allergic reaction to a previous dose of any other type of influenza vaccine is considered a precaution. RIV has similar considerations.2
Vaccines that are available for administration during the 2024-2025 season are detailed in TABLE 4.
Pregnancy
The risk of severe infection and/or complications, such as hospitalization, ICU admission, preterm delivery, and maternal/fetal death, is higher in pregnant and postpartum women. Therefore, pregnant and postpartum women should receive inactivated or recombinant influenza vaccine as soon as it is available.2,51 Vaccination during pregnancy has benefits to the newborn and reduces hospitalizations.51
Egg Allergy
Egg allergy should not preclude routine, yearly influenza vaccination. The ACIP recommends that any influenza vaccine (including egg-based) may be used if the vaccine is appropriate for age and health status.2 Per 2024-2025 recommendations, regardless of past egg reactions, no additional safety measures are required for influenza vaccination beyond those in place for any vaccine.2
Trace amounts of ovalbumin and other egg proteins may be found in egg-based and cell culture-based influenza vaccines; however, no cases of anaphylaxis or hypersensitivity reactions have been identified.2 Ovalbumin content has been reported to be less than 1 mcg/0.5 mL per dose of vaccine.2
Antiviral Pharmacotherapy for Influenza Treatment or Exposure
Last updated in 2018, guidelines from the Infectious Diseases Society of America (IDSA) detail recommendations regarding antiviral pharmacotherapy for treatment and chemoprophylaxis of influenza.52 For persons who are not at high risk of complications, the IDSA recommends antiviral treatment for those with documented or suspected influenza, regardless of vaccination history, who are 1) ill <2 days before presentation; 2) symptomatic household contacts of individuals at high risk for developing influenza complications; or 3) symptomatic healthcare providers caring for those at high risk for developing influenza complications.52
Regardless of vaccination status, antiviral therapy is recommended for immediate initiation in patients with documented or suspected influenza if they are hospitalized with influenza or are outpatients with severe or progressive illness (regardless of age), at high risk of complications, are aged younger than 2 years or older than 64 years, or are pregnant or 2 weeks postpartum.51
Antiviral therapy goals are to reduce complications and illness duration and severity.52-54 Historically, oral adamantanes (amantadine and rimantadine) were influenza treatment options. Significant drug resistance and severe side effects, however, nullified their use, and adamantanes are no longer recommended.55
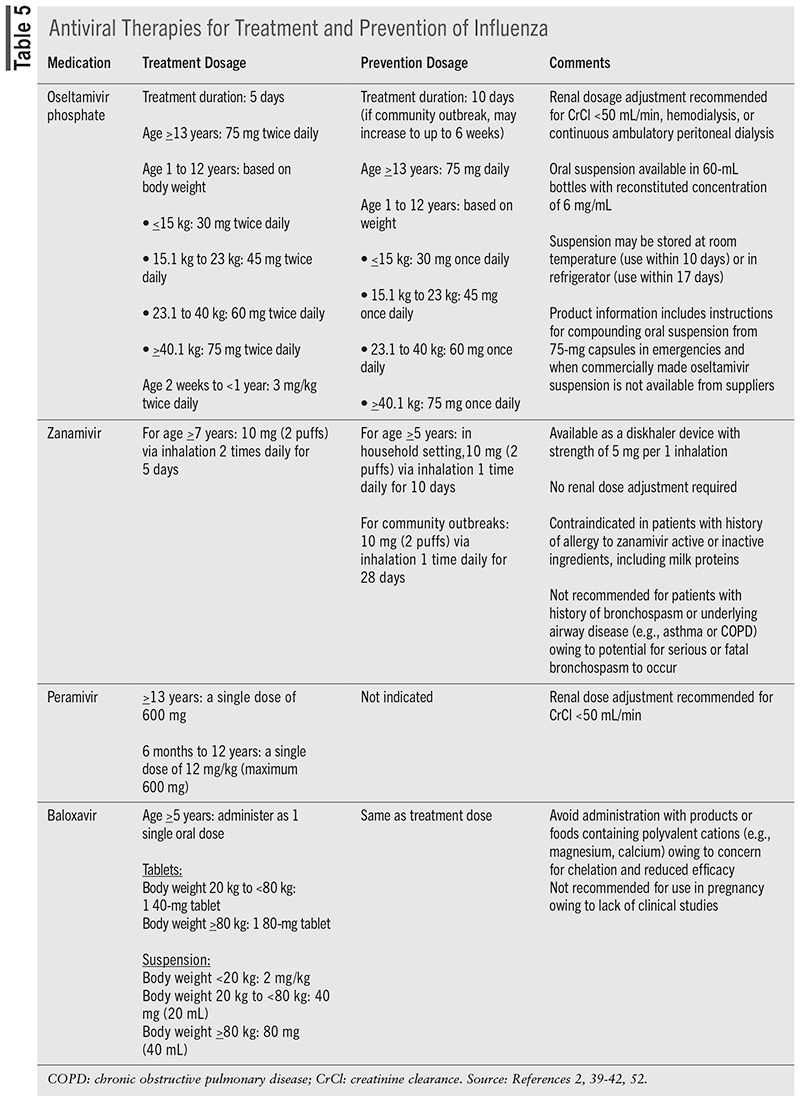
Currently, three neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir) and one capdependent endonuclease inhibitor (baloxavir) are approved antiviral therapies for influenza treatment (TABLE 5).39-42,52-55 Neuraminidase inhibitors (NAIs) prevent the release of virions from infected host cells into the respiratory epithelium by inhibiting neuraminidase enzyme. NAIs prevent cleavage of the glycosidic bond of sialic acid (SA) by competing with natural substrates for NA catalytic sites.55 Capdependent endonuclease inhibitors (CENI) affect viral transcription processes by disrupting the "cap-snatching" process. The PB1 viral subunit uses short, 5°-capped RNA oligos as primers for initiation of the transcription of influenza virus. These "caps" are generated via binding by the PB2 subunit and cleavage of pre-mRNAs by the PA subunit. CENI inhibit this process, thereby stopping viral transcription before it starts. CENI and NAI are active against both influenza A and B.55
Efficacy
NAI and CENI efficacy in influenza A and B treatment has been demonstrated.56-62 When started within 36 to 48 hours of symptom onset, NAIs decrease illness duration up to 1 day in adults and children with uncomplicated influenza.56-60 In hospitalized patients, oseltamivir treatment reduced the risk of clinical failure by 26% (95% CI 3.2%-48.0%).61 When compared with standard of care or placebo, hospital-stay duration was decreased by oseltamivir (mean difference -1.63 days, 95% CI -2.81 to -0.45) and peramivir (-1.73 days, 95% CI -3.33 to -0.13); however, the authors noted a low certainty of evidence.62 Additional observational studies in hospitalized patients found clinical benefit when starting NAI therapy within 48 hours and potentially up to 4 to 5 days after illness onset.63
The baloxavir CAPSTONE-1 trial evaluated time to symptom alleviation in outpatients aged 12 to 64 years with uncomplicated influenza (N = 1,064).64 With baloxavir, time to symptom alleviation was significantly less (53.7 hours; 95% CI 49.5-58.5) compared with placebo (80.2 hours; 95% CI 72.6-87.1) (P <.001).64 In CAPSTONE-2, baloxavir effectiveness in time to improvement of influenza symptoms was evaluated in a modified intent-to-treat population (N = 1,163) of outpatients aged >12 years with uncomplicated influenza.65 Compared with placebo, baloxavir significantly reduced time to symptom improvement (73.2 vs. 102.3 hours; P <.0001). Time to symptom improvement was comparable between baloxavir and oseltamivir.65
NAIs are effective for postexposure prophylaxis.56,66 Oseltamivir demonstrated significant overall protective efficacy in household contacts of influenza-infected cases aged >12 years of 89% for individuals and 84% for households (P <.001).66 A systematic review found that oseltamivir reduced individual risk of symptomatic influenza (risk difference [RD] 3.05%, 95% CI 1.83-3.88), with the number needed to benefit (NNTB) of 33 (range 26-55). Zanamivir also reduced individual risk (RD 1.98%; 95% CI 0.98-2.54; NNTB = 51 [40- 103]). Household risk was reduced by oseltamivir (RD 13.6%; 95% CI 9.52-15.47; NNTB = 7 [6-11]) and by zanamivir (RD 14.84%; 95% CI 12.18-16.55; NNTB = 7 [7-9]).56
Baloxavir effectively lowered influenza infection risk compared with placebo (1.9% vs. 13.6%) in 752 household contacts of 545 index patients (adjusted risk ratio 0.14; 95% CI .06-0.30; P <.001). Subgroup analysis of high-risk, pediatric, and unvaccinated individuals found a reduced risk of influenza infection when they were treated with baloxavir compared with placebo (adjusted risk ratio, 0.43; 95% CI 0.32-0.58).67
Resistance
Antiviral resistance continues to be evaluated. Globally, susceptibility to NAI therapies remains high. Global analyses of the 2018-2019 and 2019-2020 seasons found that reduced or highly reduced inhibition by NAIs was .5% (for 2018-2019 season) and .6% (for 2019- 2020 season).68
Oseltamivir
Oseltamivir has the widest age indication for influenza A and B treatment in patients who are symptomatic for no more than 48 hours and may be used in individuals aged as young as 14 days. The age range increases to 1 year and older for prophylaxis.39,43 Based on history of use, cost, and ease of administration, oseltamivir is the preferred antiviral medication for the treatment and prevention of influenza in pediatric patients.43 Oseltamivir is also the preferred antiviral therapy for pregnant and postpartum women and for hospitalized patients and outpatients with complications or progressive influenza.51,69,70
Upon oral administration of oseltamivir phosphate, hepatic esterases convert the prodrug to the active form, oseltamivir carboxylate.39,71 Oseltamivir is available in oral capsule and suspension formulations administered over 5 days for treatment and 10 days for chemoprophylaxis of influenza, and it is dose adjusted in moderate-to-severe renal impairment. For individuals aged 12 years or younger, oseltamivir is dosed based on body weight (TABLE 5). Common side effects include nausea, vomiting, and headache. Neuropsychiatric side effects, such as delirium and abnormal behavior, and serious skin/hypersensitivity reactions (e.g., Stevens-Johnson syndrome) have been reported.36 No drug-drug interactions have been identified.39
Zanamivir
Unique in its delivery system, zanamivir is an oral inhalation powder that is delivered via diskhaler device and is indicated for the treatment of influenza A and B in persons aged 7 years or older with symptoms starting no more than 2 days prior to presentation.40 Zanamivir is also indicated for persons aged 5 years or older for the prevention of influenza A and B. Zanamivir is not indicated for persons residing in nursing homes.40 No dose adjustment is required in renal/hepatic impairment. No specific drug-drug interactions have been identified. If bronchodilators are prescribed in conjunction with zanamivir, it is recommended to use the bronchodilator prior to administration of zanamivir. Side effects include dizziness, sinusitis, fever/chills, and arthralgias. Zanamivir is not recommended for use in persons with underlying airway disease owing to concern for bronchospasm. Neuropsychiatric side effects have also been reported.40
Peramivir
For persons aged 6 months and older with acute uncomplicated influenza symptomatic for no more than 2 days, peramivir is administered via IV infusion as a single dose up to 600 mg.41 While IV administration may limit its use, peramivir is also not recommended for treatment of severe influenza or for chemoprophylaxis. Trials of peramivir also primarily included patients infected with influenza A and were limited in influenza B.41
Peramivir is supplied in single-use vials of 200 mg/20 mL that must be diluted to a final concentration between 1 mg/mL and 6 mg/mL prior to administration. Infusions should be administered over 15 to 30 minutes. No specific drug-drug interactions have been identified. Common side effects include diarrhea, constipation, nausea, and vomiting. Neuropsychiatric side effects, such as delirium and abnormal behavior, and serious skin or hypersensitivity reactions (e.g., Stevens-Johnson syndrome) have also been reported.41
Baloxavir
Baloxavir marboxil, a prodrug converted to the active metabolite baloxavir, was approved in 2018 and is indicated for treatment of acute uncomplicated influenza in persons aged 5 years and older, symptomatic for no more than 48 hours, and healthy or at high risk of developing complications related to influenza.42 Baloxavir is also approved for persons aged 5 years and older for chemoprophylaxis. Baloxavir is administered orally as a single dose based on body weight. As body weight increases, baloxavir exposure is noted to decrease; however, no clinically significant difference was observed. Common side effects include vomiting, diarrhea, nausea, headache, bronchitis, and sinusitis.42 Upon reconstitution, Baloxavir oral suspension must be administered within 10 hours. Baloxavir should not be administered with foods, beverages, or other products containing polyvalent cations, such as calcium or magnesium, owing to a concern for decreased effectiveness.42
ROLE OF THE PHARMACIST
Opportunities for pharmacist involvement in influenza prevention and treatment are numerous. Studies have shown increased vaccination rates with pharmacist-provided immunization programs and pharmacist involvement in community vaccination efforts.72-75
Pharmacist-led point-of-care testing and test-to- treat programs may also reduce urgent care and emergency department burdens, improve antimicrobial stewardship, and increase access to antiviral therapy if indicated.76-79
Economic, access, and social vaccination barriers include lack of healthcare services, travel challenges, care costs, insurance, language barriers, health literacy, and medical misinformation.80 Additional barriers include missed opportunities or lack of interventions to increase vaccination demand and spur provider collaboration with other care providers.75 To address challenges in vaccination efforts and improve preparation, prevention, detection, and response to seasonal epidemics and potential pandemics, the National Influenza Vaccine Modernization Strategy (NIVMS) 2020-2030 was created.81 NIVMS strategic goals are to strengthen and diversify influenza vaccine development, manufacturing, and supply chain; promote innovative approaches and use of new technologies to detect, prevent, and respond to influenza; and increase influenza vaccine access and coverage across all populations.81
Pharmacists are well positioned to lend their expertise in reducing vaccination barriers and achieving the goals of the NIVMS. Pharmacists in any setting are poised to provide education to optimize antiviral medication use and reduce vaccine hesitancy. Pharmacists can screen patients to recommend and/or administer age-appropriate vaccines. Test-to-treat programs enable pharmacists to provide point-of-care testing for detection and prevention of influenza and other respiratory illnesses. Pharmacists may also prescribe appropriate antiviral therapy based on collaborative-practice agreements. Drug shortages may occur, and the supply of influenza vaccine or antiviral therapies may vary from season to season. Pharmacists may assist in identifying alternative options for prevention and intervention, where available.
CONCLUSION
Despite available vaccines for influenza, infection rates and costs of influenza illness remain high. Multiple types of influenza vaccines are available, and efforts to ensure adequate supply and vaccine efficacy and tolerability continue. Current antivirals, NAIs and baloxavir, are safe and effective in the treatment and chemoprophylaxis of influenza. As an integral part of the healthcare team and highly accessible community healthcare provider, the pharmacist is well poised to lend their expertise in vaccination and treatment efforts for each influenza season and in the prevention and management of potential future pandemics.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
- Hall E. Influenza. In: Hall E, Wodi AP, Hamborsky J, et al, eds. Epidemiology and Prevention of Vaccine-Preventable Diseases. 14th ed. CDC. Washington, DC: Public Health Foundation; 2021.
- Grohskopf LA, Ferdinands JM, Blanton LH, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices-United States, 2024- 25 influenza season. MMWR Recomm Rep. 2024;73(5):1-25.
- CDC. Preliminary estimated flu disease burden 2024-2025 flu season. www.cdc.gov/flu-burden/php/data-vis/2024-2025.html. Accessed May 29, 2025.
- Putri WCWS, Muscatello DJ, Stockwell MS, Newall AT. Economic burden of seasonal influenza in the United States. Vaccine. 2018;36(27):3960-3966.
- Olsen SJ, Winn AK, Budd AP, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic-United States, 2020-2021. MMWR Morb Mortal Wkly Rep. 2021;70(29):1013-1019.
- CDC. Flu disease burden: past seasons. www.cdc.gov/flu-burden/php/data-vis/past-seasons.html. Accessed May 29, 2025.
- Van Wormer JJ, King JP, Gajewski A, et al. Influenza and workplace productivity loss in working adults. J Occup Environ Med. 2017;59(12):1135-1139.
- Blanchet Zumofen MH, Frimpter J, Hansen SA. Impact of influenza and influenza-like illness on work productivity outcomes: a systematic literature review. Pharmacoeconomics. 2023;41:253-273.
- Petrie JG, Cheng C, Malosh RE, et al. Illness severity and work productivity loss among working adults with medically attended acute respiratory illnesses: US Influenza Vaccine Effectiveness Network 2012-2013. Clin Infect Dis. 2016;62(4):448-455.
- Neuzil KM, Hohlbein C, Zhu Y. Illness among schoolchildren during influenza season: effect on school absenteeism, parental absenteeism from work, and secondary illness in families. Arch Pediatr Adolesc Med. 2002;156(10):986-991.
- Zviedrite N, Jahan F, Zheteyeva Y, et al. School closures due to seasonal influenza: a prospective data collection-based study of eleven influenza seasons-United States, 2011-2022. Lancet Reg Health Am. 2024;34:100741.
- World Health Organization. Clinical Practice Guidelines for Influenza. Geneva, Switzerland; 2024. Licence: CC BY-NC-SA 3.0 IGO.
- Layton C, Lenfestey N. Influenza vaccine manufacturing. Issue Brief. October 2005. RTI Project Number 0208665.001. www.rti.org/sites/default/files/resources/laytonmanufacturing.pdf. Accessed May 29, 2025.
- Pérez Rubio A, Eiros JM. Cell culture-derived flu vaccine: present and future. Hum Vaccin Immunother. 2018:14(8):1874-1882.
- Cell culture as a substrate for the production of influenza vaccines: memorandum from a WHO meeting. Bull World Health Organ. 1995;73(4):431-435
- Cox MM. Recombinant protein vaccines produced in insect cells. Vaccine. 2012;30(10):1759-1766.
- Jarvis DJ. Baculovirus-insect cell expression systems. In: Burgess RR, Deutscher MP, eds. Methods of Enzymology. San Diego, CA: Academic Press; 2009;463:191-222.
- Van Oers M. Vaccines for viral and parasitic diseases produced with baculovirus vectors. Adv Virus Res. 2006;68:193-253.
- Flublok product information. Meriden, CT: Protein Sciences; 2024.
- World Health Organization. Recommended composition of the influenza virus vaccines for use in the 2024 southern hemisphere influenza season. www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2024-southern-hemisphere-influenza-season. Accessed May 29, 2025.
- FDA. Influenza vaccine composition for the 2025-2026 U.S. influenza season. www.fda.gov/vaccines-blood-biologics/influenza-vaccine-composition-2025-2026-us-influenza-season. Accessed May 29, 2025.
- Ray GT, Lewis N, Klein NP, et al. Intraseason waning of influenza vaccine effectiveness. Clin Infect Dis. 2019;68(10):1623-1630.
- Domnich A, Orsi A, Signori A, et al. Waning intra-season vaccine effectiveness against influenza A(H3N2) underlines the need for more durable protection. Expert Rev Vaccines. 2024;23(1):380-388.
- Belongia EA, Sundaram ME, McClure DL, et al. Waning vaccine protection against influenza A (H3N2) illness in children and older adults during a single season. Vaccine. 2015;33(1):246-251.
- Lee JKH, Lam GKL, Shin T, et al. Efficacy and effectiveness of high-dose influenza vaccine in older adults by circulating strain and antigenic match: an updated systematic review and meta-analysis. Vaccine. 2021;39(Suppl 1):a24-a35.
- Skaarup KG, Lassen MCH, Modin D, et al. The relative vaccine effectiveness of high-dose vs standard-dose influenza vaccines in preventing hospitalization and mortality: a meta-analysis of evidence from randomized trials. J Infect. 2024;89:106187.
- Shay DK, Chillarige Y, Kelman J, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries in preventing postinfluenza deaths during 2012-2013 and 2013-2014. J Infect Dis. 2017;215(4):510-517.
- Young-Xu Y, Snider JT, van Aalst R, et al. Analysis of relative effectiveness of high-dose versus standard-dose influenza vaccines using an instrumental variable method. Vaccine. 2019;37(11):1484-1490.
- Young-Xu Y, Van Aalst R, Mahmud SM, et al. Relative vaccine effectiveness of high-dose versus standard-dose influenza vaccines among Veterans Health Administration patients. J Infect Dis. 2018;217:1718-1727.
- Young-Xu Y, Snider JT, Mahmud SM, et al. High-dose influenza vaccination and mortality among predominantly male, white, senior veterans, United States, 2012/13 to 2014/15. Euro Surveill. 2020;25:1900401.
- Ko EJ, Kang SM. Immunology and efficacy of MF59-adjuvanted vaccines. Hum Vaccin Immunother. 2018;14(12):3041-3045.
- Pelton SI, Divino V, Shah D, et al. Evaluating the relative vaccine effectiveness of adjuvanted trivalent influenza vaccine compared to high-dose trivalent and other egg-based influenza vaccines among older adults in the US during the 2017-2018 influenza season. Vaccines (Basel). 2020;8(3):446.
- Pelton SI, Divino V, Postma MJ, et al. A retrospective cohort study assessing relative effectiveness of adjuvanted versus high-dose trivalent influenza vaccines among older adults in the United States during the 2018-19 influenza season. Vaccine. 2021;39(17):2396-2408.
- Flumist product information. Gaithersburg, MD: MedImmune; 2024.
- CDC. Live attenuated influenza vaccine [LAIV] (the nasal spray flu vaccine). www.cdc.gov/flu/vaccine-types/nasalspray.html. Accessed May 29, 2025.
- FDA. FDA approves nasal spray influenza vaccine for self- or caregiver-administration. September 20, 2024. www.fda.gov/news-events/press-announcements/fda-approves-nasal-spray-influenza-vaccine-self-or-caregiver-administration. Accessed May 29, 2025.
- Jhaveri R, Allyne K. A feasibility trial of home administration of intranasal vaccine by parents to eligible children. Clin Ther. 2017;39(1):204-211.e4.
- Burgess TH, Murray CK, Bavaro MF, et al. Self-administration of intranasal influenza vaccine: immunogenicity and volunteer acceptance. Vaccine. 2015;33(32):3894-3899.
- Tamiflu (oseltamivir phosphate) product information. South San Francisco, CA: Genentech, Inc.; 2019.
- Relenza (zanamivir inhalation powder) product information. Durham, NC: GlaxoSmithKline; 2023.
- Rapivab (peramivir) product information. Durham, NC: BioCryst Pharmaceuticals, Inc.; 2024.
- Xofluza (baloxavir marboxil) product information. South San Francisco, CA: Genentech, Inc.; 2024.
- American Academy of Pediatrics, Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2024-2025: policy statement. Pediatrics. 2024;154(4):e2024068507.
- Afluria product information. Parkville, Victoria, Australia: Seqirus; 2024.
- Fluarix product information. Dresden, Germany: GlaxoSmithKline; 2024.
- Flucelvax product information. Holly Springs, NC: Seqirus; 2024.
- FluLaval product information. Quebec City, QC, Canada: ID Biomedical Corporation of Quebec; 2024.
- Fluzone product information. Swiftwater, PA: Sanofi Pasteur; 2024.
- Fluzone High-Dose product information. Swiftwater, PA: Sanofi Pasteur: 2024.
- Fluad product information. Holly Springs, NC: Seqirus; 2024.
- American College of Obstetricians and Gynecologists. Influenza in pregnancy: prevention and treatment. Obstet Gynecol. 2024;143(2):e24- e30. www.acog.org/clinical/clinical-guidance/committee-statement/articles/2024/02/influenza-in-pregnancy-prevention-and-treatment. Accessed May 29, 2025
- Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis. 2019;68:895-902.
- Gaitonde DY, Moore FC, Mackenzie MK. Influenza: diagnosis and treatment. Am Fam Phys. 2019;100(12):751-758.
- CDC. Treating flu with antiviral drugs. www.cdc.gov/flu/treatment/antiviral-drugs.html. Accessed May 29, 2025.
- Bonomini A, Mercorelli, B, Loregian A. Antiviral strategies against influenza virus: an update on approved and innovative therapeutic approaches. Cell Mol Life Sci. 2025;82(1):75.
- Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in adults and children. Cochrane Database Syst Rev. 2014;(4):CD008965.
- Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385(9979):1729-1737.
- Malosh RE, Martin ET, Heikkinen T, et al. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis. 2018;66(10):1492-1500.
- Liu JW, Lin SH, Wang LC, et al. Comparison of antiviral agents for seasonal influenza outcomes in healthy adults and children: a systematic review and network meta-analysis. JAMA Netw Open. 2021;4(8):e2119151.
- Tejada S, Jansson M, Solé-Lleonart C, Rello J. Neuraminidase inhibitors are effective and safe in reducing influenza complications: meta-analysis of randomized controlled trials. Eur J Intern Med. 2021;86:54-65.
- Wiemken TL, Furmanek SP, Carrico RM, et al. Effectiveness of oseltamivir treatment on clinical failure in hospitalized patients with lower respiratory tract infection. BMC Infect Dis. 2021;21:1106.
- Gao Y, Guyatt G, Uyeki TM, et al. Antivirals for treatment of severe influenza: a systematic review and network meta-analysis of randomised controlled trials. Lancet. 2024;404(10454):753-763.
- CDC. Influenza antiviral medications: summary for clinicians. www.cdc.gov/flu/hcp/antivirals/summary-clinicians.html#Table1. Accessed May 29, 2025.
- Hayden FG, Sugaya N, Hirotsu N, et al; Baloxavir Marboxil Investigators Group. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med. 2018;379(10):913-923.
- Ison MG, Portsmouth S, Yoshida Y, et al. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2020;20(10):1204-1214.
- Welliver R, Monto AS, Carewicz O, et al; Oseltamivir Post Expo- sure Prophylaxis Investigator Group. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. JAMA. 2001;285(6):748-754.
- Ikematsu H, Hayden FG, Kawaguchi, et al. Baloxavir marboxil for prophylaxis against influenza in household contacts. N Engl J Med. 2020;383:309-320.
- Govorkova EA, Takashita E, Daniels RS, et al. Global update on the susceptibilities of human influenza viruses to neuraminidase inhibitors and the cap-dependent endonuclease inhibitor baloxavir, 2018-2020. Antiviral Res. 2022;200:105281.
- American Academy of Pediatrics, Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2024-2025: policy statement. Pediatrics. 2024;154(4):e2024068507.
- CDC. Recommendations for obstetric health care providers related to use of antiviral medications for the treatment and prevention of influenza. www.cdc.gov/flu/hcp/antivirals/treatment_obstetric.html. Accessed May 29, 2025.
- CDC. Influenza antiviral medications: summary for clinicians. www.cdc.gov/flu/hcp/antivirals/summary-clinicians.html. Accessed May 29, 2025.
- Davies BE. Pharmacokinetics of oseltamivir: an oral antiviral for the treatment and prophylaxis of influenza in diverse populations. J Antimicrob Chemother. 2010;65(Suppl 2):ii5-ii10.
- Papastergiou J, Folkins C, Li W, Zervas J. Community pharmacist-administered influenza immunization improves patient access to vaccination. Can Pharm J. 2014;147(6):359-365.
- Le LM, Veettil SK, Donaldson D, et al. The impact of pharmacist involvement on immunization uptake and other outcomes: an updated systematic review and meta-analysis. J Am Pharm Assoc (2003). 2022;62(5):1499-1513.e16.
- Murray E, Bieniek K, del Aguila M, et al. Impact of pharmacy intervention on influenza vaccination acceptance: a systematic literature review and meta-analysis. Int J Clin Pharm. 2021;43:1163-1172.
- Elghanam Y, Kim EY. Impact of pharmacist intervention on enhancing vaccination coverage: a systematic review and meta-analysis. Res Social Admin Pharm. 2025;21(7):495-504.
- Hohmeier KC, McKeirnan K, Akers J, et al. Implementing community pharmacy-based influenza point-of-care test-and-treat under collaborative practice agreement. Implement Sci Commun. 2022;3:77.
- Hardin R, Roberts P, Hudspeth B, et al. Development and implementation of an influenza point-of-care testing service in a chain community pharmacy setting. Pharmacy. 2020;8(4):182.
- Herbin SR, Klepser DG, Klepser ME. Pharmacy-based infectious disease management programs incorporating CLIA-waived point-of- care tests. J Clin Microbiol. 2020;58(5):e00726-19.
- CDC. Ensuring vaccine access for all people. www.cdc.gov/vaccines/basics/vaccine-equity.html. Accessed May 29, 2025.
- National Influenza Vaccine Modernization Strategy (NIVMS): 2020-2030. https://aspr.hhs.gov/legal/NIVMS/Pages/default.aspx. Accessed May 29, 2025.