Management of Male Urinary Incontinence
RELEASE DATE
June 1, 2025
EXPIRATION DATE
June 30, 2027
FACULTY
Donna M. Lisi, PharmD, BS Pharm, BCPS, BCGP,
BCACP, BCPP, BCMTMS, FASCP, FAAPP
Clinical Pharmacist
Somerset, New Jersey
FACULTY DISCLOSURE STATEMENTS
Dr. Lisi has no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-25-042-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To educate pharmacists about the management of male urinary incontinence (UI).
EDUCATIONAL OBJECTIVES
After completing this activity, the participant should be able to:
- Describe the pathophysiology of male UI and the different types of UI in men.
- Define the risk factors for male UI, including the role of medication in contributing to male UI.
- Identify both pharmacologic and nonpharmacologic interventions in the management of urge UI and stress UI in men.
- Discuss the role that pharmacists can play in optimizing the management of male UI.
ABSTRACT: Urinary incontinence (UI), which is the involuntary loss of urine experienced during the bladder storage phase of micturition, occurs in almost 40% of men aged older than 60 years. The pathophysiology of male UI is complex and involves the central nervous system and the urinary tract. Key considerations include the mechanisms and differing types of UI in men, risk factors, diagnosis, and both nonpharmacologic and pharmacologic interventions. Pharmacists can play a major role in optimizing the care of men with UI by conducting medication reviews to avoid drugs that can exacerbate UI and by counseling patients on the proper use of both nonpharmacologic and pharmacologic interventions.
Urinary incontinence (UI) is defined as the involuntary loss of urine experienced during the bladder storage phase of micturition.1 Differences in pathophysiology and prevalence exist between the five types of UI (stress, urge, mixed, overflow, and functional) among men and women.2 It is important to distinguish between the types of UI, as treatment options differ.3
PATHOPHYSIOLOGY OF MALE UI
UI results from a complex interaction between the central nervous system (CNS; brain and signal transmission) and the urinary tract (UT), including the internal urethral sphincter (IUS), the external urethral sphincter (EUS), the urethra, and the bladder neck.1,3
Normal Micturition
The bladder maintains a low intravesical pressure (<20 cm H2O) until it fills or the detrusor muscle (DM), which is the smooth muscle layer that forms the main wall of the urinary bladder, contracts, expelling urine during urination. This happens in coordination with the urethral sphincters. Relaxation of the DM allows for bladder filling and storage of urine, which stimulates sensory nerves or stretch receptors in the bladder wall. The urethral muscles consist of the EUS and IUS.1,4
The EUS is innervated by the autonomic and somatic nervous system. The IUS is primarily innervated by the autonomic nervous system, specifically through both the sympathetic and parasympathetic branches; it functions involuntarily. The sympathetic nervous system is responsible for the contraction of the IUS, which prevents the loss of urine, while the parasympathetic nervous system helps relax the IUS to facilitate urination. Also involved in micturition is the somatic nervous system, which regulates the EUS to maintain voluntary control over urination. Parasympathetic postganglionic nerve terminals release acetylcholine, which interacts with muscarinic receptors to produce bladder contractions. Sympathetic postganglionic terminals release norepinephrine, which produces contractions of the bladder base and urethral smooth muscle and relaxation of the bladder body.1,5
UI and frequency occur when there is loss of bladder compliance (i.e., the ability of the bladder to manage increasing amounts of urine without increasing pressure). Conditions that are associated with a loss of compliance are neurologic conditions (e.g., multiple sclerosis [MS], cauda equina syndrome), DM overactivity, DM instability, radiation effects, chronic denervation, long-term bladder outlet obstruction (BOO), and infectious and noninfectious cystitis.1,4
There is an interrelatedness between urge UI (UUI) and overactive bladder (OAB), as OAB can present with nocturia with or without UUI. Also, OAB can lead to UUI.1,6 UUI is also correlated with benign prostatic hyperplasia (BPH), nonneurogenic lower UT symptoms (LUTS), and neurogenic lower UT dysfunction (NLUTD). LUTS encompasses both storage and voiding symptoms, whereas UUI is primarily related to storage symptoms, such as urgency and frequency; however, when these storage symptoms become severe enough to cause involuntary urine leakage, it can indicate the presence of OAB. Therefore, LUTS, especially the storage-type symptoms, are strongly linked to UUI with or without the presence of OAB.3,7,8
Pathophysiologic Mechanisms of UI
Detrusor Overactivity or Detrusor Underactivity: Normal detrusor function allows the bladder to fill with little or no change in pressure. The urge to urinate occurs when there is approximately 150 mL to 200 mL of urine in the bladder; by the time the bladder contains 300 mL to 400 mL of urine, there is a sensation that the bladder is full and detrusor overactivity (DO; hyperactivity) occurs as involuntary contractions of the bladder, causing it to empty prematurely. Detrusor underactivity can also lead to UI owing to inadequate bladder contractions, which prevent full emptying, resulting in urine leakage when the bladder becomes overfilled. DO may be due to neurogenic causes or idiopathic.1,4,9
BPH and OAB: As men age, they develop benign prostatic enlargement (BPE) that can result in BOO, which is blockage to urinary flow or benign prostatic obstruction (BPO). BPO and BPE can lead to LUTS, resulting in leakage of urine. LUTS can form independently of BPH and may be due to other medical conditions, such as UT infections (UTIs) or OAB. DO, impaired compliance, and UUI occur in BPO. LUTS in BPH presents as either storage disorders, which manifest as urinary frequency, nocturia, urgency, or UUI, or voiding disorders (i.e., weak stream, straining, and incomplete emptying), which can cause incomplete bladder emptying. OAB involves storage symptoms, and patients with BPH can also develop OAB symptoms, leading to UI due to the impact of the enlarged prostate on the bladder’s function.1,8,10,11
OAB resulting from primary DO or underactivity manifests as storage symptoms, but OAB may also result from BPE and BPO secondary to obstruction. Treatment options vary depending on the pathophysiological process involved in UI.1,8,10,11
Post Prostate Treatment Incontinence: The prevalence of post radical prostatectomy UI, which ranges from 40% (e.g., based on monitoring the number of absorbent pads used or leakage into a pad after 1 hour) to 80% (when self-reporting) is dependent on the assessment instrument used and the type of surgical procedure.12,13
During a radical prostatectomy (RP), the IUS is resected, affecting innervation and resulting in impaired detrusor contractility. Any physiological compromise or insult to the sphincters, their supporting structures (as in the case of a prostatectomy or surgery for BPH), and/or neural innervation can result in UI.1,3
Neurogenic Urinary Incontinence: Neurologic pathology that leads to UI is divided into upper motor neuron lesions, which include the cerebral, brain, and spinal cord lesions, and lower motor neuron lesions, which include the cauda equina and peripheral nerves. Neurogenic DO may be due to MS, spinal cord injury (SCI), and myelodysplasia in young adults. Other neurogenic conditions that can lead to UI include stroke, cerebral infarction, Parkinson’s disease (PD), multiple system atrophy, dementia, and diabetes.1,14
TYPES OF URINARY INCONTINENCE
There are five major types of UI in men and women, although the frequencies of occurrence and etiologies may vary by sex (see TABLE 1).2,3,15-18 Guidelines from both the International Continence Society and the European Association of Urology (EAU), however, subdivide UI into only three major groups: UUI, stress UI (SUI), and mixed UI (MUI).1,3
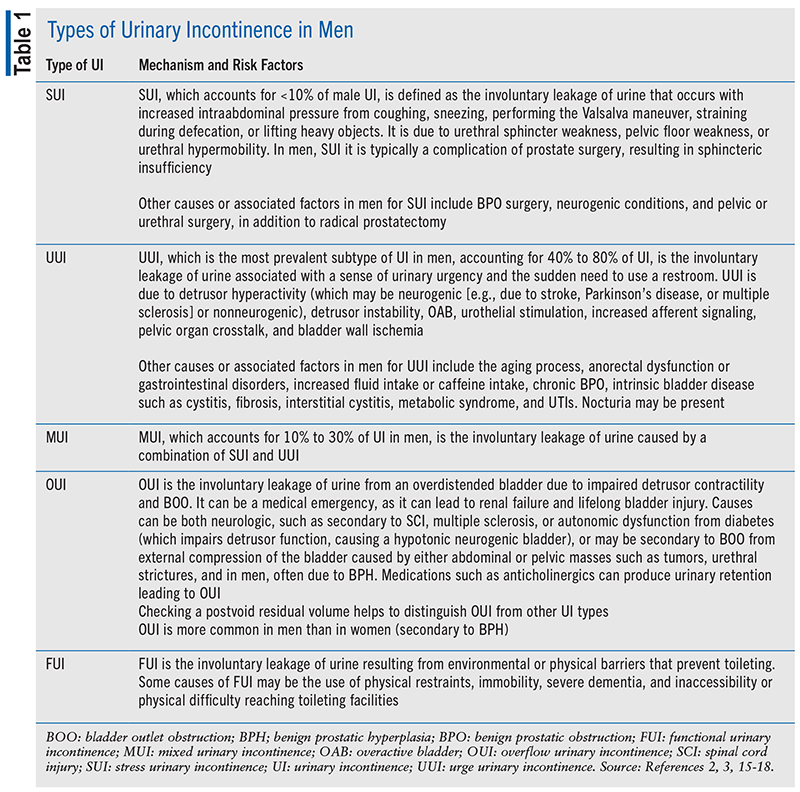
EPIDEMIOLOGY OF MALE UI
There is a paucity of epidemiological data on male UI. Data from the 2001–2020 National Health and Nutrition Examination Survey found that 38.5% (with UUI accounting for 31.3%) of men aged >60 years suffer from UI. As UUI and overflow UI (OUI) have increased, there has been a trend for a decrease in SUI, especially among those aged >60 years. This decrease in SUI is attributed to a decline in RPs. The increase in OUI may be secondary to increased rates of BPH, especially among black and Hispanic men.19
OAB significantly increased from 11.3% in 2005–2008 to 14.5% by 2015–2020. Higher rates were seen in white and black males aged 40 to 59 years who were overweight or obese.20
COMMON RISK FACTORS FOR MALE UI
A scoping review involving 47 articles was conducted to identify risk factors (RFs) for UI in older men. Six categories of RFs were described: medical factors/diseases (two most common were BPH/prostate problems and diabetes); environmental factors (e.g., being institutionalized, use of physical restraints); physiological RFs and age-related physiological changes (e.g., decreased functional ladder capacity/increased postvoid residual volume [PVRV]); behavioral factors (e.g., tobacco, alcohol, caffeine, or bladder irritant use); and other (e.g., DO/detrusor instability, limitation in physical function/activities of daily living limitations/low composite physical performance score, medications/polypharmacy). From these six categories, 98 RFs were identified.21
Two of the most common causes of male UI are incontinence after prostate treatment (IPT) and medications.15,22,23
INCONTINENCE AFTER PROSTATE TREATMENT
IPT can occur following an RP, radiation therapy, and treatment of BPH. The American Urological Association (AUA) issued guidelines on the management of IPT. While IPT generally resolves to near baseline by 12 months postprocedure, it may persist. Patients should be counseled on the risk of UI. It is important to distinguish between UI due to sphincteric insufficiency (which is indicative of SUI) or bladder dysfunction (which is indicative of UUI). The prevalence of OAB in this patient population is as high as 48%.15
MEDICATIONS
There are several mechanisms by which medications can increase the risk of UI.22 Drug-induced SUI may be due to 1) decreases in urethral sphincter tone, which may result from alpha-adrenoreceptor inhibition or 2) a complex interaction involving central and peripheral serotoninergic, muscarinic, or gabaminergic receptors, leading to urine leakage secondary to adverse effects on sphincter pressure. Drugs and/or drug classes that are associated with SUI include antipsychotics, alpha-adrenergic antagonists (e.g., prazosin, doxazosin, terazosin, phenoxybenzamine, and phentolamine), benzodiazepines and Z-sedative hypnotics, and misoprostol.23
Medication-related UUI may occur via several mechanisms, including 1) an increase in involuntary detrusor contractility during the storage phase of micturition secondary to stimulation of muscarinic receptors and 2) resulting from complex action on central and peripheral serotonergic receptors or estrogenic/progesteronic receptors in the urethra, vesical triangle, sacrouterine ligament, levator ani muscles, and pubocervical fascia. Drugs associated with UUI are acetylcholinesterase inhibitors, tricyclic antidepressants (e.g., amitriptyline, nortriptyline, desipramine), serotonin 5-hydroxytryptamine(4) receptor agonists (e.g., cisapride, prucalopride), and estrogen/progesterone preparations.23
Drug-mediated OUI may be secondary to urinary retention that causes a buildup of intravesical pressure that exceeds sphincter pressure, leading to urine leakage. Drugs that induce urinary retention include antimuscarinic agents, antihistamines, anti-PD agents, beta-adrenergic agonists, calcium channel blockers, opioids, and sedatives. Drugs that block the renin-angiotensin system (i.e., angiotensin-converting enzyme inhibitors and angiotensin receptor antagonists) can also contribute to the development of OUI by decreasing both detrusor contractility and urethral sphincter tone, causing the bladder to not empty fully due to reduced detrusor contraction. The bladder becomes overfilled, and the sphincter pressure is exceeded. SUI is also associated with an increase in abdominal pressure from coughing and sneezing.23
Other substances, such as alcohol, caffeine, and diuretics, can produce excess urine by inhibiting reabsorption of fluid and electrolytes or by increasing the glomerular filtration rate, causing the bladder to fill more rapidly and increasing the frequency of urination.23
Use of proton pump inhibitors (PPIs) was found to be statistically significantly associated with a 36% increased risk of developing OAB, although causality was not established. The frequency of urinary symptoms increased significantly by 3% per year with continued PPI use. Proposed mechanisms of PPI-induced urinary symptoms include alterations of the gut microbiome, interference with the body’s oxidative stress response, and reduction in acid-dependent nutrient absorption (e.g., magnesium) that is needed to maintain nerve and muscle function. Gut microbiome alterations may also affect sex hormone metabolism, further adversely affecting bladder function.24
The scoping review of UI in older men identified hypnotics/sedatives, anticholinergics, androgen deprivation therapy for prostate cancer, diuretics, CNS depressants, and alpha-antagonists or -agonists as RFs, although alpha-antagonists were useful in men with an enlarged prostate to prevent BOO and OUI.21
Other RFs for male IU include, but are not limited to, neurologic diseases (e.g., UI is increased in men following a stroke, with >50% of stroke patients experiencing UI, especially following a cortical stroke); toxic exposures (e.g., mono-3-carboxypropyl phthalate, lead); substances of abuse (e.g., cotinine; marijuana, which increases the risk of developing the urinary condition by 39% and whose use is not supported in the management of OAB); vitamin D deficiency (e.g., increases the risk for SUI in older men); poor general health; physical disabilities; cognitive impairment; UTIs; advancing age and frailty; higher weight; oxidative stress; and sleep apnea.25-40
DIAGNOSIS
Obtaining a medical history, including details on the timing and severity of UI and other urinary symptoms, comorbidities, and medications, and a physical examination to check for the presence of a distended bladder, enlarged prostate, or evidence of abdominal or pelvic masses is strongly recommended. A digital rectal examination is also useful.3
There are validated questionnaires to help capture and quantify the severity of UI symptoms (e.g., the International Consultation on Incontinence Questionnaire–Short Form). These questionnaires screen for and categorize UI as well as assess its impact on quality of life.41-47
Voiding diaries provide a measure of the frequency and extent of UI episodes and can be used both therapeutically and to monitor the effects of interventions.3
Pad tests quantify the number of incontinence pads a patient uses to determine the severity of UI and to monitor response to treatment; however, the usefulness of the pad test in determining the outcome of treatment has been questioned.3
A PVRV is used to determine the ability of the bladder to empty and the presence of urinary retention and to evaluate for the presence of detrusor dysfunction. Uroflowmetry assesses peak and average flow rates, total void time, and total void volume, with peak urine flow rates <10 mL/sec to 12 mL/sec indicative of obstruction. These tests may be performed as part of the workup for BPH and for OUI.8,48
Imaging studies, including magnetic resonance imaging or computed tomography scan, and urinalysis can be performed to check for anatomical and functional abnormalities or the presence of a UTI, respectively.3
GUIDELINES ON MALE UI
Guidelines on management of UI in men are available from the EAU and are based on the complete EAU guideline on nonneurogenic male LUTS.3,17
The AUA has information on male UI in several different guidelines, including IPT, which primarily presents as SUI and to a lesser extent UUI and urgency-predominant MUI; BPH, which presents as OUI and UUI; OAB, which is associated with UUI; and neurogenic lower tract dysfunction, which can be associated with UUI, OUI, and functional UI.7,9,14-16,49 The AUA has also disseminated guidelines on the management of SUI; however, these recommendations are specific to SUI in females.50
GENERAL MANAGEMENT
It is important to find out what matters most to patients, as one study indicated that while dryness was important to men with UI, other factors, such as simplicity of treatment options, the need for future retreatment, treatment satisfaction, and avoiding surgery, were also important points to consider.51
Lifestyle Modifications
Lifestyle modifications are recommended for all forms of male IU, although the evidence supporting this intervention is lacking. These interventions include losing weight if overweight or obese, quitting smoking, increasing the level of physical activity, and eating healthy (e.g., consuming a diet rich in anthocyanidin and flavone, which can be found in fruits, vegetables, and plant-derived beverages, such as tea and juice).3,52
Vitamin D supplementation produced no to mixed efficacy results for improving OAB or UI in men.53
Treatment of Comorbidities
It is important to manage comorbidities that may be contributing to UI. Medications can contribute to the development of UI. Fluid overload from heart failure and the subsequent use of diuretics may exacerbate UI.3 The presence of constipation may increase intraabdominal pressure; however, there is no evidence that treating constipation improves UI.54,55
Pads and Devices (Containment)
Absorbent incontinence pads or adult diapers are categorized as containment products. Other products that fall under this category include urinary catheters for intermittent catheterization, external collection devices, and penile clamps. Combinations of these devices can be used based on need.3 A penile sheath, or condom catheter, is a soft, flexible sleeve that fits over the penis with an antireflux bulbous end that culminates as a short tube and attaches to any standard urinary drainage system.56 Penile-compression devices are useful to manage postprostatectomy UI; however, these devices should not be used in men with cognitive impairment, problems with dexterity, or those lacking sensation.57
The AUA guidelines on the management of incontinence post prostatectomy recommend absorbent pads, penile compression devices (clamps), and catheters as first-line approaches to manage UI.15,16
Behavioral Strategies
Behavioral modification includes prompted or time voiding, bladder training, and pelvic floor–muscle training. Behavioral strategies have been found to be very helpful in managing UI in men. In prompted voiding, patients are prompted to void rather than initiating it themselves. With timed voiding, patients are using the toilet on a fixed schedule. These strategies may be especially useful for patients with cognitive impairment and UI.3
The goal of bladder training is to improve the abnormal patterns of frequent urination and poor bladder control and to empower patients to regain bladder function. Bladder training may be used in conjunction with anticholinergic therapy.3
One of the most useful strategies for managing UI is pelvic floor–muscle training. When used alone or together with biofeedback and/or electrical stimulation for post prostatectomy UI, it was found to be very effective in improving the time to gain continence; however, data at 12 months postsurgery failed to demonstrate long-term benefit.3
AUA guidelines on IPT recommend offering pelvic floor–muscle exercises prior and immediately after RP; pelvic floor–muscle training can also be offered in the immediate postoperative period.15
A Cochrane Database systematic review found that based on low to very low evidence, bladder training may cure or improve OAB compared with no treatment.58
Neuromodulation
Neuromodulation can be either surface or intra-anal electrostimulation or posterior tibial nerve stimulation. When combined with pelvic floor–muscle training, improvement is seen at 6 months but not at 12 months. Both strategies demonstrate significant improvement in frequency, urgency, and urgency episodes over a 3-month period.3
PHARMACOLOGIC MANAGEMENT OF UI
Pharmacologic Agents for UUI With or Without OAB
This section will examine UUI with and without OAB. It will pull from various guidelines, including the EUA’s and the AUA’s guidelines on BPH, OAB, and neurogenic lower tract dysfunction.3,7,8,14,17,49 A review on the management of BPH was recently published.60 There are no sex-specific treatment recommendations from the AUA on OAB.61 UUI is managed pharmacologically with the use of either muscarinic receptor antagonists (MRAs) or beta-3 receptor agonists (beta-3 RAs).62,63
Muscarinic Receptor Antagonists
MRAs act by blocking the action of acetylcholine at the muscarinic receptors, specifically at the M2 and M3 receptors, which are found on the DM. This leads to prevention of involuntary detrusor contractions, increased bladder capacity, and decreased episodes of UI.62,63 Available MRAs to treat UUI include oxybutynin, tolterodine, darifenacin, fesoterodine, solifenacin, flavoxate, and trospium chloride.64
The AUA guidelines on idiopathic OAB emphasize that MRAs were associated with increased risk of all-cause dementia and Alzheimer’s disease and recommend that cognitive risk be considered prior to prescribing these medications long-term.49 The guidelines state that clinicians should counsel patients on the side effects of medications used to treat the condition and that patients should engage in shared decision-making. It especially highlights that there is evidence suggesting a cumulative and dose-dependent association between the use of MRAs and the development of new-onset dementia.49 A review was recently published on assessing anticholinergic/antimuscarinic effects in older adults.65
These drugs should be used with extreme caution in patients with OAB who have narrow-angle glaucoma, impaired gastric emptying, or a history of urinary retention. Gastric emptying may be impaired in patients with diabetes, those who had prior abdominal surgery, those receiving opioids, and those with scleroderma, hypothyroidism, PD, or MS. In patients with a history of urinary retention or who are at risk of new-onset urinary retention, a PVRV should be obtained prior to starting these medications. Antimuscarinic therapy should be assessed within 1 to 2 months to determine both efficacy and the occurrence of adverse drug effects. If improvement is not observed, therapy should be changed; however, data are limited supporting substituting agents within the same class.49 All MRAs are not created equally, as oxybutynin appears to have a greater risk of dementia than darifenacin or solifenacin, which are more uroselective muscarinic agents, when these agents are used short-term.66
The AUA BPH guidelines recommend that anticholinergic agents, used alone or in combination with an alpha-blocker, may be offered to patients with moderate-to-severe predominant storage LUTS in BPH.7,8
The AUA NLUTD guidelines recommend the use of MRAs or beta-3 RAs, or a combination of both, to improve bladder storage parameters.14 The EAU guidelines on male UI recommend the use of MRAs as monotherapy since they significantly improve urgency and UUI and can reduce daytime frequency.3 These drugs are also effective in men with OAB who have no evidence of BOO.8,10,11
A Cochrane Database systematic review found that based on low to very low evidence, bladder training may be more effective than MRAs for the management of OAB and is associated with fewer adverse events.58
The New South Wales Therapeutic Advisory Group Inc. (NSWTAG) has developed a two-step approach to deprescribing anticholinergic drugs (antimuscarinics) used for UI. Among the indications for deprescribing urinary MRAs are the lack of an appropriate indication for use; presence of adverse drug events; drug-drug or drug-disease interactions; high drug burden; nonadherence; and patient preference. Anticholinergic burden should also be considered. The NSWTAG guide provides steps on how to wean anticholinergic drugs, which should be reduced slowly by 25% to 50% of the daily dose every 1 to 4 weeks. Adjustments should be made based on response. If the patient is tolerating the drug withdrawal, then continue to complete drug withdrawal. If there is worsening confusion, the guideline recommends to abruptly discontinue. The NSWTAG also recommends considering a slower taper (e.g., 12.5%) during the final 2 weeks of deprescribing when reducing to the final dose. If dosage forms are limited to make the appropriate dose reductions, consider using an alternate-day dosing schedule. Patients need to be closely monitored during the deprescribing process. If there is a recurrence of symptoms or the presence of withdrawal symptoms, reverting to the previous lowest tolerated dose may be helpful. Attempts to deprescribe may be tried after 6 to 12 weeks at the lower weaning rate (e.g., 5%-12.5% of the daily dose each month), followed by totally withdrawing the drug.67
The latest version of the STOPP/START criteria (Version 3) and the Beers criteria for potentially inappropriate medications have identified several drugs used in the management of UI that may be potentially inappropriate in older adults (see TABLES 2 and 3).68,69
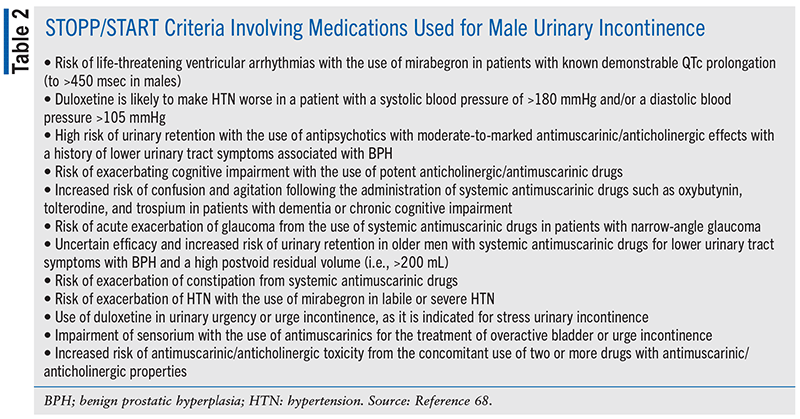
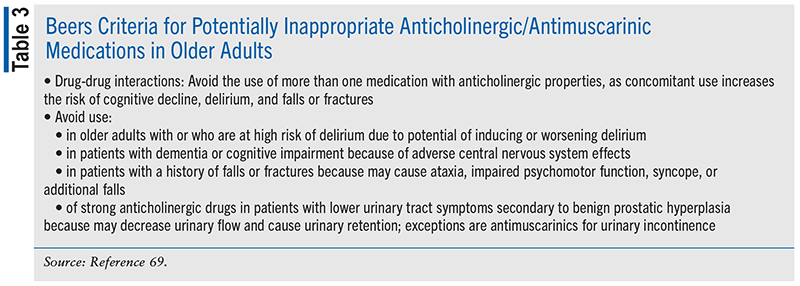
Despite the warnings about the risks associated with the use of MRAs, a study conducted in the long-term care setting found that 75% of directors of nursing were not aware of a link between anticholinergic medications and risk of cognitive adverse effects.70
Beta-3 RAs
Stimulation of beta-3 receptors relaxes the detrusor smooth muscle during the storage phase of the urinary bladder fill-void cycle, which increases bladder capacity. There are two FDA-approved beta-3 RAs, mirabegron and vibegron, both of which are indicated for the management of OAB.71
The AUA guidelines on BPH recommend that beta-3 RAs in combination with an alpha-blocker may be offered to patients with moderate-to-severe storage-predominant LUTS.7,8 The AUA guidelines on idiopathic OAB recommend a trial of a beta-3 RA before MRAs. Beta-3 RAs appear to have lower rates of adverse events. It is recommended to switch to a beta-3 RA, especially if tolerability is an issue, since both drugs are efficacious. If monotherapy with an OAB medication does not achieve an adequate response, combining medications from a different class is recommended. Beta-3 RAs are used to improve urinary urgency, frequency, and/or urge UI.49
The EAU guidelines on male UI offer a strong recommendation for the use of either MRAs or mirabegron to manage male patients with UI who have failed conservative treatment.3 They state that mirabegron is superior to placebo and as efficacious as MRAs for the improvement of UUI. Vibegron is not mentioned in the EAU guidelines.3 The AUA guidelines on idiopathic OAB consider mirabegron and vibegron to be equally effective, although the latter does not carry the hypertension warning and precaution that the former’s product labeling has, and it may have a slightly faster onset of effect, but data are limited in men.49
Several studies have investigated the use of beta-3 RAs in the management of urinary symptoms, including UI and OAB in men.72-74 In a 12-week postmarketing surveillance study, mirabegron was found to be effective (based on Overactive Bladder Symptom Score) in improving urinary symptoms in men with OAB whether or not they had BPH; however, the effect on UI was not reported. In patients with BPH and OAB, there is concern that patients may experience an increased PVRV with mirabegron use.73 Mirabegron 50 mg daily was found to be safe and effective in reducing urgency and UI when administered to frail patients with OAB who were aged older than 80 years (median age 85 years; 65% male).75 It may be preferred over the use of MRAs in men with OAB.76
There are little data comparing mirabegron 25 mg/day to 50 mg/day with vibegron 75 mg/day in OAB. In a study involving mostly women, vibegron was associated with significant improvement in total incontinence episodes compared with mirabegron 50 mg (and tolterodine 4 mg extended-release) and in the volume voided per micturition. There was a suggestion of a more rapid onset of efficacy with vibegron compared with mirabegron at 4 weeks. Hypertension was the most common adverse event for both mirabegron and vibegron; vibegron was also commonly associated with UTIs.77
Vibegron is associated with a significant reduction in mean daily micturitions, urgency episodes, nocturia episodes, UUI episodes, International Prostate Symptom Score storage scores, and volume voided per micturition compared with placebo in men treated for OAB associated with BPH. Although the adverse event rates were similar between vibegron and placebo, the most common adverse events were hypertension, COVID-19, and hematuria.74
The results have been mixed on the benefit associated with the use of mirabegron when added to alpha-blockers in men with OAB. A post hoc analysis of the MATCH study found that mirabegron 50 mg added to tamsulosin did not significantly improve UI compared with placebo and tamsulosin in men with OAB.78 A pooled analysis of four trials, however, found that mirabegron added to tamsulosin significantly improved UUI but was associated with an increase in the PVRV.79 In a 12-week direct comparison of tamsulosin 0.4 mg plus mirabegron 50 mg once daily versus tamsulosin 0.4 mg plus solifenacin 5 mg once daily in men with persistent OAB, both combinations were found to be effective, but the antimuscarinic combination was associated with more adverse events (10.9% vs. 26.1%, respectively), including dry mouth, constipation, and increased PVRV.80 A systematic review and network meta-analysis found no statistically significant difference in efficacy and safety between the use of an alpha-1 blocker plus mirabegron versus an alpha-1 blocker plus an MRA in men with LUTS secondary to BPH and OAB, but numerical differences were observed in favor of safety for mirabegron (i.e., in treatment-emergency adverse events, urinary retention, and Qmax) and in favor of efficacy for the MRA (e.g., micturitions/day).81
TABLE 4 lists the drugs used for male UUI with or without OAB.82
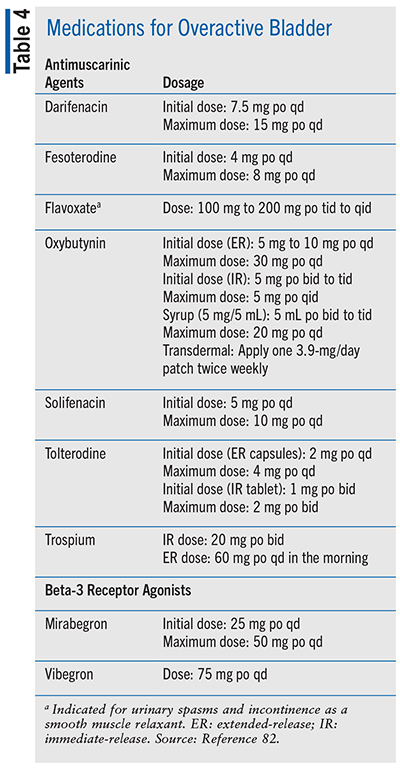
Pharmacologic Agents for SUI (Including Incontinence After Prostate Treatment)
Duloxetine: According to EAU guidelines on male UI, the pharmacotherapeutic recommendation for the management of SUI is duloxetine. Duloxetine is associated with short-term improvement in postprostatectomy SUI symptoms and quality-of-life improvement, but a significant proportion of men discontinued treatment. Patients should be informed about the possible adverse events of duloxetine. Additionally, duloxetine is not FDA-labeled for use in UI.3
A recent systematic review on duloxetine for postprostatectomy SUI found that while duloxetine demonstrated good short-term cure and/or improvement in UI and in reducing time to attainment of continence following surgery, 38% of patients discontinued therapy due to adverse events or lack of efficacy.83
The AUA guidelines on the management of SUI are specific to SUI in females and therefore not applicable.83 Further, the AUA guideline on IPT, which is primarily associated with SUI, does not include any pharmacologic agents in the management of IPT.15,16
Surgical Technique: A detailed discussion of surgical techniques is beyond the scope of this article. Focus will be limited to botulinum toxin type A (onabotulinumtoxinA) for UUI.3
UUI Surgical Treatment: Surgical treatment of UUI involves bladder wall injection of onabotulinumtoxinA, sacral nerve stimulation or neuromodulation, and cystoplasty/urinary diversion.3
OnabotulinumtoxinA: OnabotulinumtoxinA acts by blocking neuromuscular transmission at motor or autonomic nerve terminals and inhibiting the release of acetylcholine.84
OnabotulinumtoxinA is the only botulinum toxin approved for use for genitourinary conditions in the United States, including for the treatment of UI due to DO associated with a neurologic condition or for treatment of OAB with symptoms of UI, urgency, and frequency in adults who have not had an adequate response to or are not tolerant to other treatment modalities.85,86 TABLE 5 includes dosing information for onabotulinumtoxinA for bladder dysfunction.
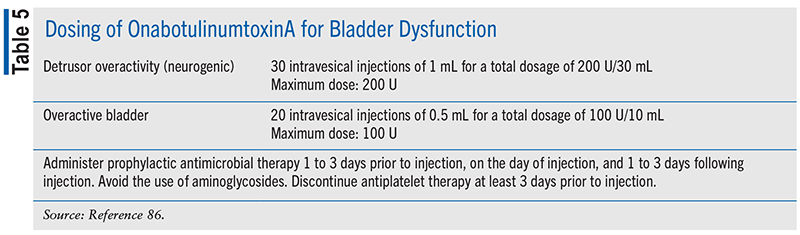
The AUA guidelines on NLUTD recommend that for patients with SCI or MS refractory to oral medications, clinicians should recommend onabotulinumtoxinA to improve bladder storage parameters, decrease episodes of incontinence, and improve quality-of-life measures. Further, in patients with NLUTD dysfunction other than those with SCI and MS or who are refractory to oral medications, clinicians may offer onabotulinumtoxinA to improve bladder storage parameters, decrease episodes of incontinence, and improve quality-of-life measures.14
The EAU makes the following recommendations regarding the use of onabotulinumtoxinA:
- A single treatment session of onabotulinumtoxinA (100 U) injected in the bladder wall is more effective than placebo at curing and improving UUI/OAB symptoms and quality of life.
- There is no evidence that repeated injections of onabotulinumtoxinA have reduced efficacy, but discontinuation rates are high.
- There is an increased risk of retention and UTIs with onabotulinumtoxinA injections.3
Most studies of the use of onabotulinumtoxinA have involved the management of UI primarily in women.87-93 Several studies, however, have examined the role of onabotulinumtoxinA in men for the management of UI.61,94-98
One study identified predictors of adverse events in men following the administration of onabotulinumtoxinA. Having a high PVRV and an onabotulinumtoxinA dose of >100 U was associated with the need for intermittent self-catheterization after onabotulinumtoxinA injection. Others have found that the PVRV may stay elevated for 2 weeks.94
Several studies have examined the role of onabotulinumtoxinA for male UI secondary to neurogenic DO.96 One study demonstrated that there was improvement in UI episodes and in urodynamic assessment in men with neurogenic DO. However, hematuria, urinary retention, and autonomic dysreflexia occurred in 9% of patients.96 Another study found that less than one-half continued botulinum injections long-term (i.e., up to 7 years). The disadvantage of having to intermittently self-catheterize made onabotulinumtoxinA a less attractive therapeutic option.97
There is a paucity of data on onabotulinumtoxinA use in male patients with OAB. This is problematic because the differences in male and female pathophysiology could possibly affect the outcome of treatment of OAB. OnabotulinumtoxinA is most effective in men with a small prostate and low prostate-specific antigen levels.61,98 Whereas UTIs may be a more common adverse effect in women following onabotulinumtoxinA injections, hematuria appears to be more common in males. Urinary retention may also be slightly higher in the latter group.61
Mixed UI
Patients with MUI should be managed via the AUA’s OAB guidelines.49
COMPLEMENTARY AND ALTERNATIVE/INTEGRATIVE MEDICINE
Acupuncture: Much of the literature on the use of acupuncture in the management of UI is in the postprostatectomy setting; however, acupuncture is not addressed in the IPT guidelines, nor is the guideline mentioned in the EUA’s guidelines on male UI or nonneurogenic male LUTS.3,17,99
The theoretical basis for the potential benefit of electroacupuncture is that stimulation of the BL33 and BL25 acupuncture sites, both of which are in the lumbosacral region, and stimulation of the SP6 acupuncture site, which is the posterior tibial region, may result in sacral neuromodulation and regulation of bladder function.100
A recent systematic review on acupuncture for postprostatectomy incontinence found the available literature to be of low quality and had a high degree of bias.100 The latest Cochrane Database systematic review on acupuncture for OAB found that the evidence is “very uncertain about the effect acupuncture has on cure or improvement of OAB symptoms compared to no treatment.” These findings were not specific to the male population.101 The National Center for Complementary and Integrative Health indicates that acupuncture may be beneficial in women with UI, however, no data are presented on male UI.102
Other Complementary and Alternative Medicines: The AUA guidelines on OAB state that clinicians should counsel patients that there is currently insufficient evidence to support the use of nutraceuticals, vitamins, supplements, or herbal remedies in the treatment of patients with OAB.49
COMPLICATIONS
Several complications related to the presence of UI in males include incontinence-associated dermatitis, a specific type of irritant contact dermatitis that is characterized by the presence of erythema and edema of the perianal or genital skin that may also be associated with bullae, erosion, or secondary cutaneous infection; depression, suicidal ideation, and social isolation; increased fall risk (up to 88% higher in men with UI); increased mortality; UTIs; loss of dignity; loss of finance; sexual dysfunction and maintenance of relationships; loss of independence.35,103-121
PHARMACIST’S ROLE IN MANAGING MALE UI
There is a paucity of information on the pharmacist’s role in the management of UI, in particular on male UI. One retrospective, observational, before-and-after, community-based study conducted by the Programs of All-Inclusive Care for the Elderly found that over a 9-month period, 63.1% of deprescribing recommendations for urinary MRAs were implemented. Standardized daily doses of MRAs decreased by 65.4%.122
A second, small study that took place in long-term care facilities that involved 14 residents in the intervention group (and 10 residents in the control group) showed that eight of the residents in the intervention group reported decreased anticholinergic adverse effects upon deprescribing of their MRA, but four residents reported worsening of their OAB symptoms.123
Unlike the U.S., pharmacists in the United Kingdom are at the forefront of providing continence care in the community as part of its Pharmacy Role In proMotion of continencE (PRIME) study.124-126 Another community pharmacy initiative in the UK was designed to support men after prostate cancer treatment.127
As the population ages, pharmacists can play a vital role in educating men with UI about OTC products (e.g., continence undergarments and pads), conducting medication review to minimize exposure to drugs that can exacerbate symptoms of UI, and monitoring for adverse effects of medications used for UI.
REFERENCES
- Cardozo L, Rovner E, Wagg A, et al. Incontinence. 7th ed. Bristol, UK: International Continence Society; 2023.
- Leslie SW, Tran LN, Puckett Y. Urinary incontinence. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2025 Jan-.
- Gacci M, Sakalis VI, Karavitakis M, et al. European Association of Urology guidelines on male urinary incontinence. Eur Urol. 2022;82(4):387-398.
- Thomas L. Micturition reflex—neural control of urination. News Medical Life Sciences. April 5, 2023. www.news-medical.net/health/Micturition-Reflex-Neural-Control-of-Urination.aspx. Accessed May 12, 2025.
- Yoshimura N, Chancellor MB. Neurophysiology of lower urinary tract function and dysfunction. Rev Urol. 2003;5(Suppl 8):s3-s10.
- Steers WD. Pathophysiology of overactive bladder and urge urinary incontinence. Rev Urol. 2002;4(Suppl 4):s7-s18.
- Sandhu JS, Bixler BR, Dahm P, et al. Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia (BPH): AUA guideline amendment 2023. J Urol. 2024;211(1):11-19.
- Lerner LB, McVary KT, Barry MJ, et al. Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline part I—initial work-up and medical management. J Urol. 2021;206(4):806-817.
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61(1):37-49.
- Kim MK, Shin YS, Lee JH, et al. The prevalence of lower urinary tract symptoms and overactive bladder in South Korea: a cross-sectional, population-based study. Int Neurourol J. 2022;26(1):31-36.
- Ng M, Leslie SW, Baradhi KM. Benign prostatic hyperplasia. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024 Jan-.
- Mata LRFD, Azevedo C, Izidoro LCR, et al. Prevalence and severity levels of post-radical prostatectomy incontinence: different assessment instruments. Rev Bras Enferm. 2021;74(2):e20200692.
- Licari LC, Bologna E, Manfredi C, et al. Postoperative urinary incontinence following BPH surgery: insights from a comprehensive national database analysis. Minerva Urol Nephrol. 2024;76(5):618-624.
- Ginsberg DA, Boone TB, Cameron AP, et al. The AUA/SUFU guideline on adult neurogenic lower urinary tract dysfunction. J Urol. 2021;206(5):1097-1105.
- Sandhu JS, Breyer B, Comiter C, et al. Incontinence after prostate treatment: AUA/SUFU guideline. J Urol. 2019;202(2):369-378.
- Breyer BN, Kim SK, Kirkby E, et al. Updates to incontinence after prostate treatment: AUA/GURS/SUFU guideline (2024). J Urol. 2024;212(4):531-538.
- European Association of Urology. EAU guidelines on non-neurogenic male lower urinary tract symptoms (LUTS). https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Non-Neurogenic-Male-LUTS-2025.pdf. Accessed February 15, 2025.
- Dunkin MA. Overflow incontinence. WebMD. August 31, 2024. www.webmd.com/urinary-incontinence-oab/overflow-incontinence. Accessed February 15, 2025.
- Cao C, Zhang C, Sriskandarajah C, et al. Trends and racial disparities in the prevalence of urinary incontinence among men in the USA, 2001–2020. Eur Urol Focus. 2022;8(6):1758-1767.
- Cheng Y, Chen T, Zheng G, et al. Prevalence and trends in overactive bladder among men in the United States, 2005–2020. Sci Rep. 2024;14(1):16284.
- Olagundoye O, Odusanya B, Kung JY, et al. A scoping review of risk factors for urinary incontinence in older men. BMC Geriatr. 2023;23(1):534.
- Tsakiris P, Oelke M, Michel MC. Drug-induced urinary incontinence. Drugs Aging. 2008;25(7):541-549.
- Dobrek L. Lower urinary tract disorders as adverse drug reactions–a literature review. Pharmaceuticals (Basel). 2023;16(7):1031.
- Du YZ, Liu JH, Zheng FC, et al. Association between proton pump inhibitor use and overactive bladder risk in adults: a cross-sectional study. Urology. 2024;194:67-74.
- Uysal İ, Doğrukök ÖN, Golcuk Y, et al. Urinary incontinence in men with stroke: a cross-sectional study. Medicina (Kaunas). 2025;61(1):52.
- Fluck A, Fry CH, Affley B, et al. Sex-specific independent risk factors of urinary incontinence in acute stroke patients: a multicentre registry-based cohort study. Neurourol Urodyn. 2024;43(4):818-825.
- Khan J, Shaw S. Risk of multiple lower and upper urinary tract problems among male older adults with type-2 diabetes: a population-based study. Aging Male. 2023;26(1):2208658.
- Pop-Busui R, Braffett BH, Wessells H, et al. Diabetic peripheral neuropathy and urological complications in type 1 diabetes: findings from the epidemiology of diabetes interventions and complications study. Diabetes Care. 2022;45(1):119-126.
- Yi X, Jin K, Qiu S, et al. Phthalate exposure enhances incidence of urinary incontinence: US NHANES, 2003–2004 and 2005–2006. Environ Sci Pollut Res Int. 2022;29(43):64692-64703.
- Fu M, Zhu Z, Xiang Y, et al. Associations of blood and urinary heavy metals with stress urinary incontinence risk among adults in NHANES, 2003–2018. Biol Trace Elem Res. 2025;203(3):1327-1341.
- Shi C, Yang L, Zeng G, et al. Association between serum cotinine levels and urinary incontinence in adults in the United States: a population-based cross-sectional analysis. BMC Public Health. 2024;24(1):2326.
- Zhu S, Wang Z, Tao Z, et al. Relationship between marijuana use and overactive bladder (OAB): a cross-sectional research of NHANES 2005 to 2018. Am J Med. 2023;136(1):72-78.
- Liu L, Xu M, Zhou H, et al. Association of serum 25-hydroxyvitamin D with urinary incontinence in elderly men: evidence based on NHANES 2007–2014. Front Endocrinol (Lausanne). 2023;14:1215666.
- Yuan P, Wang T, Li H, et al. Systematic review and meta-analysis of the association between vitamin D status and lower urinary tract symptoms. J Urol. 2021;205(6):1584-1594.
- Yates A. Addressing the gender gap in urinary continence care. Br J Nurs. 2023;32(Supp19):s11-s16.
- Cao S, Hu X, Tang Y, et al. Weight-adjusted-waist index is positively associated with urinary incontinence: results from the National Health and Nutrition Examination Survey (NHANES) 2001–2018. Eur J Med Res. 2024;29(1):368.
- Chen F, Lin H, Zhang Y, et al. Impact of weight loss on the risk of urinary incontinence: the role of sex and body type. World J Urol. 2024;42(1):616.
- Xu Z, Elrashidy RA, Li B, Liu G. Oxidative stress: a putative link between lower urinary tract symptoms and aging and major chronic diseases. Front Med (Lausanne). 2022;9:812967.
- Li B, Li F, Xie X, et al. Associations between obstructive sleep apnea risk and urinary incontinence: insights from a nationally representative survey. PLoS One. 2024;19(11):e0312869.
- Shahait M, Nguyen TT, Asmar J, et al. Impact of obstructive sleep apnea syndrome on time to complete recovery of continence after robot-assisted radical prostatectomy: a propensity score matching analysis. J Endourol. 2023;37(8):882-888.
- ICIQ.net. International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form (ICIQ-UI SF). https://iciq.net/iciq-ui-sf. Accessed May 12, 2025.
- Avery K, Donovan J, Peters T, et al. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23(4):322-230.
- Coyne K, Revicki D, Hunt T, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res. 2002;11(6):563-574.
- National Multiple Sclerosis Society. Bladder control scale (BLCS). www.nationalmssociety.org/for-professionals/for-researchers/researcher-resources/research-tools/clinical-study-measures/blcs. Accessed May 12, 2025.
- MDCalc. Revised urinary incontinence scale (RUIS). www.mdcalc.com/calc/2047/revised-urinary-incontinence-scale-ruis. Accessed May 12, 2025.
- Margolis MK, Vats V, Coyne KS, Kelleher C. Establishing the content validity of the King’s Health Questionnaire in men and women with overactive bladder in the US. Patient. 2011;4(3):177-187.
- Robinson JP, Shea JA. Development and testing of a measure of health-related quality of life for men with urinary incontinence. J Am Geriatr Soc. 2002;50(5):935-945.
- FDA. Non-clinical and clinical investigation of devices used for the treatment of benign prostatic hyperplasia (BPH). December 27, 2021. www.fda.gov/media/79397/download. Accessed May 12, 2025.
- Cameron AP, Chung DE, Dielubanza EJ, et al. The AUA/SUFU guideline on the diagnosis and treatment of idiopathic overactive bladder. J Urol. 2024;212(1):11-20.
- Kobashi KC, Vasavada S, Bloschichak A, et al. Updates to surgical treatment of female stress urinary incontinence (SUI): AUA/SUFU guideline (2023). J Urol. 2023;209(6):1091-1098.
- 51. Hampson LA, Shaw NM, Breyer BN, et al. Patient-identified treatment attributes among older men with stress urinary incontinence: a qualitative look at what matters to patients making treatment decisions. Urology. 2023;177:189-196.
- Lin C, Lyu J, Feng Z. Intake of dietary flavonoids in relation to overactive bladder among U.S. adults: a nutritional strategy for improving urinary health. Front Nutr. 2024;11:1437923.
- Markland AD, Vaughan CP, Huang AJ, et al. Effect of vitamin D supplementation on overactive bladder and urinary incontinence symptoms in older men: ancillary findings from a randomized trial. J Urol. 2023;209(1):243-252.
- Son YJ, Kwon BE. Overactive bladder is a distress symptom in heart failure. Int Neurourol J. 2018;22(2):77-82.
- National Association for Continence. Can constipation cause urinary incontinence? www.nafc.org/bhealth-blog/can-constipation-cause-urinary-incontinence/. Accessed May 12, 2025.
- Kyle G. The use of urinary sheaths in male incontinence. Br J Nurs. 2011;20(6):338.
- Shaw C, Wagg A. Penile compression devices for the treatment of urinary incontinence: current status and future prospects. Expert Rev Med Devices. 2024;21(9):811-817.
- Funada S, Yoshioka T, Luo Y, et al. Bladder training for treating overactive bladder in adults. Cochrane Database Syst Rev. 2023;10(10):CD013571.
- Yang JM, Ye H, Long Y, et al. Effect of pelvic floor muscle training on urinary incontinence after radical prostatectomy: an umbrella review of meta-analysis and systematic review. Clin Rehabil. 2023;37(4):494-515.
- Lisi DM. Managing benign prostatic hyperplasia. US Pharm. 2024;49(6):44-57.
- Nitti VW, Kohan A, McCammon K, et al. Efficacy and safety of onabotulinumtoxinA for the treatment of overactive bladder in men and women: a pooled analysis. Neurourol Urodyn. 2024;43(8):1765-1775.
- Athanasopoulos A, Giannitsas K. An overview of the clinical use of antimuscarinics in the treatment of overactive bladder. Adv Urol. 2011;2011:820816.
- Hegde SS. Muscarinic receptors in the bladder: from basic research to therapeutics. Br J Pharmacol. 2006;147(Suppl 2):s80-s87.
- UptoDate. Lexidrug. Anticholinergic agents. Accessed February 15, 2025.
- Lisi DM. Assessing anticholinergic effects in older adults. In: Update in Geriatrics. IntechOpen.com. 2021.
- Bell B, Avery A, Bishara D, et al. Anticholinergic drugs and risk of dementia: time for action? Pharmacol Res Perspect. 2021;9(3):e00793.
- New South Wales Therapeutic Advisory Group Inc. (NSW TAG). Deprescribing guide for anticholinergic drugs for urinary incontinence (antimuscarinics). www.nswtag.org.au/wp-content/uploads/2018/06/1.6-Deprescribing-Guide-for-Anticholinergic-drugs-for-Urinary-Incontinence-Antimuscarinics.pdf. Accessed May 12, 2025.
- O’Mahony D, Cherubini A, Guiteras AR, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 3. Eur Geriatr Med. 2023;14(4):625-632.
- American Geriatrics Society Beer Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081.
- Stefanacci RG, Yeaw J, Shah D, et al. Impact of urinary incontinence related to overactive bladder on long-term care residents and facilities: a perspective from directors of nursing. J Gerontol Nurs. 2022;48(7):38-46.
- Sun K, Wang D, Wu G, et al. Mirabegron improves the irritative symptoms caused by BCG immunotherapy after transurethral resection of bladder tumors. Cancer Med. 2021;10(21):7534-7541.
- Mullen GR, Kaplan SA. Efficacy and safety of mirabegron in men with overactive bladder symptoms and benign prostatic hyperplasia. Curr Urol Rep. 2021;22(1):5.
- Takahashi S, Kato D, Tabuchi H, Uno S. Safety and effectiveness of mirabegron in male patients with overactive bladder with or without benign prostatic hyperplasia: a Japanese post-marketing study. Low Urin Tract Symptoms. 2021;13(1):79-87.
- Staskin D, Owens-Grillo J, Thomas E, et al. Efficacy and safety of vibegron for persistent symptoms of overactive bladder in men being pharmacologically treated for benign prostatic hyperplasia: results from the phase 3 randomized controlled COURAGE trial. J Urol. 2024;212(2):256-266.
- Nakagomi H, Mitsui T, Shimura H, et al. Mirabegron for overactive bladder in frail patients 80 years or over (HOKUTO study). BMC Urol. 2022;22(1):40.
- De Nunzio C, Brucker B, Bschleipfer T, et al. Beyond antimuscarinics: a review of pharmacological and interventional options for overactive bladder management in men. Eur Urol. 2021;79(4):492-504.
- Kennelly MJ, Rhodes T, Girman CJ, et al. Efficacy of vibegron and mirabegron for overactive bladder: a systematic literature review and indirect treatment comparison. Adv Ther. 2021;38:5452-5464.
- Kakizaki H, Lee KS, Katou D, et al. Mirabegron add-on therapy to tamsulosin in men with overactive bladder: post hoc analyses of efficacy from the MATCH study. Adv Ther. 2021;38(1):739-757.
- Wu Y, Li G, Zhou H, et al. Mirabegron add-on tamsulosin for men with overactive bladder symptoms: a pooled analysis of four randomized controlled trials. Urol Int. 2024;108(2):118-127.
- Soliman MG, El-Abd SA, Tawfik AM, et al. Efficacy and safety of mirabegron versus solifenacin as additional therapy for persistent OAB symptoms after tamsulosin monotherapy in men with probable BPO. World J Urol. 2021;39(6):2049-2054.
- Herschorn S, Tarcan T, Jiang YH, et al. Safety and efficacy of an α1-blocker plus mirabegron compared with an α1-blocker plus antimuscarinic in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia and overactive bladder: a systematic review and network meta-analysis. Neurourol Urodyn. 2024;43(3):604-619.
- UptoDate. Lexidrug. Various drugs for urinary incontinence. Accessed February 15, 2025.
- Kobashi KC, Vasavada S, Bloschichak A, et al. Updates to surgical treatment of female stress urinary incontinence (SUI): AUA/SUFU guideline (2023). J Urol. 2023;209(6):1091-1098.
- Botox (onabotulinumtoxinA) injection, powder, lyophilized, for solution prescribing information. North Chicago, IL: AbbVie, Inc.; November 2023.
- Chancellor MB, Smith CP. Use of botulinum toxin in the genitourinary system. Handb Exp Pharmacol. 2021;263:171-184.
- UptoDate Inc. LexiDrug. Botulinum toxin products. Accessed February 15, 2025.
- Chow PM, Trump T, Goldman HB. Outcomes of sequential third-line therapies in patients with refractory overactive bladder. Int J Urol. 2024;31(7):772-777.
- Yokoyama O, Honda M, Yamanishi T, et al. Efficacy and safety of onabotulinumtoxinA in patients with overactive bladder: subgroup analyses by sex and by serum prostate-specific antigen levels in men from a randomized controlled trial. Int Urol Nephrol. 2021;53(11):2243-2250.
- DiCarlo-Meacham AM, Dengler KL, Welch EK, et al. Reduced versus standard intradetrusor onabotulinumtoxinA injections for treatment of overactive bladder. Neurourol Urodyn. 2023;42(1):366-374.
- Hamid R, Lorenzo-Gomez MF, Schulte-Baukloh H, et al. OnabotulinumtoxinA is a well tolerated and effective treatment for refractory overactive bladder in real-world practice. Int Urogynecol J. 2021;32(1):65-74.
- Schulte-Baukloh H, Weiß C, Weinberger S, et al. Real-time documentation of the effect of onabotulinumtoxin A detrusor injection in OAB patients−preliminary results. Toxins (Basel). 2022;15(1):30.
- Chen YH, Kuo JH, Huang YT, et al. Evaluating the efficacy and safety of botulinum toxin in treating overactive bladder in the elderly: a meta-analysis with trial sequential analysis of randomized controlled trials. Toxins (Basel). 2024;16(11):484.
- McCammon K, Gousse A, Kohan A, et al. Early and consistent improvements in urinary symptoms and quality of life with onabotulinumtoxinA in patients with overactive bladder and urinary incontinence: results from a randomized, placebo-controlled, phase IV clinical trial. Female Pelvic Med Reconstr Surg. 2021;27(7):450-456.
- Gotoh D, Torimoto K, Takamatsu N, et al. Intravesical injection of onabotulinumtoxinA (botulinum toxin type A) in Japanese patients with refractory overactive bladder. In Vivo. 2024;38(3):1332-1337.
- Ribeiro L, Leung LY, Tan N, et al. Predictors for adverse events following intravesical botulinum toxin injections in men. Neurourol Urodyn. 2023;42(7):1499-1505.
- Honda M, Yokoyama O, Takahashi R, et al. Botulinum toxin injections for Japanese patients with urinary incontinence caused by neurogenic detrusor overactivity: clinical evaluation of onabotulinumtoxinA in a randomized, placebo-controlled, double-blind trial with an open-label extension. Int J Urol. 2021;28(9):906-912.
- Chen SF, Jiang YH, Jhang JF, Kuo HC. Satisfaction with detrusor onabotulinumtoxinA injections and conversion to other bladder management in patients with chronic spinal cord injury. Toxins (Basel). 2022;14(1):35.
- Lee HY, Kuo HC. Intravesical injection of botulinum toxin type A in men without bladder outlet obstruction and post-deobstructive prostate surgery. Toxins (Basel). 2023;15(3):221.
- American Urological Association. Incontinence after prostate treatment: AUA/GURS/SUFU GUIDELINE (2019; amended 2024). https://auau.auanet.org/sites/default/files/media/2025-02/Incontinence%20after%20Prostate%20Treatment%20AUAGURSSUFU%20Guideline.pdf. Accessed May 12, 2025.
- Chen H, Liu Y, Wu J, et al. Acupuncture for postprostatectomy incontinence: a systematic review. BMJ Support Palliat Care. 2023;13(e1):e10-e19.
- Hargreaves E, Baker K, Barry G, et al. Acupuncture for treating overactive bladder in adults. Cochrane Database Syst Rev. 2022;9(9):CD013519.
- National Center for Complementary and Integrative Health. Acupuncture: effectiveness and safety. October 2022. www.nccih.nih.gov/health/acupuncture-effectiveness-and-safety. Accessed May 12, 2025.
- Beeckman D, Van den Bussche K, Alves P, et al. The Ghent Global IAD Categorisation Tool (GLOBIAD). Skin Integrity Research Group–Ghent University 2017. https://images.skintghent.be/20184916028778_globiadenglish.pdf. Accessed May 12, 2025.
- Koloms K, Cox J, VanGilder CA, Edsberg LE. Incontinence management and pressure injury rates in US acute care hospitals: analysis of data from the 2018–2019 International Pressure Injury Prevalence (IPUP) survey. J Wound Ostomy Continence Nurs. 2022;49(5):405-415.
- Kayser SA, Koloms K, Murray A, et al. Incontinence and incontinence-associated dermatitis in acute care: a retrospective analysis of total cost of care and patient outcomes from the premier healthcare database. J Wound Ostomy Continence Nurs. 2021;48(6):545-552.
- Deprez J, Ohde N, Eilegård Wallin A, et al. Prognostic factors for the development of incontinence-associated dermatitis (IAD): a systematic review. Int Wound J. 2024;21(7):e14962.
- Wu S, Wu F. Association of urinary incontinence with depression among men: a cross-sectional study. BMC Public Health. 2023;23(1):944.
- Filipas DK, Labban M, Beatrici E, et al. Association of urinary incontinence and depression: findings from the National Health and Nutrition Examination Survey. Urology. 2023;181:11-17.
- Zuluaga L, Caicedo JI, Mogollón MP, et al. Anxiety and depression in association with lower urinary tract symptoms: results from the COBaLT study. World J Urol. 2023;41(5):1381-1388.
- Przydacz M, Skalski M, Sobanski J, et al. Association between lower urinary tract symptoms and sleep quality of patients with depression. Medicina (Kaunas). 2021;57(4):394.
- Chen J, Liu Z, Yang L, et al. Sleep-related disorders and lower urinary tract symptoms in middle-aged and elderly males: a cross-sectional study based on NHANES 2005−2008. Sleep Breath. 2024;28(1):359-370.
- Pan T, Zhang Z, He T, et al. The association between urinary incontinence and suicidal ideation: findings from the National Health and Nutrition Examination Survey. PLoS One. 2024;19(5):e0301553.
- Moon S, Chung HS, Kim YJ, et al. The impact of urinary incontinence on falls: a systematic review and meta-analysis. PLoS One. 2021;16(5):e0251711.
- Liu PS, Huang HK, Ding DC. Association of lower urinary tract symptoms and hip fracture in adults aged ≥ 50 years. PLoS One. 2021;16(3):e0246653.
- Schluter PJ, Askew DA, Jamieson HA, Arnold EP. Urinary and fecal incontinence are independently associated with falls risk among older women and men with complex needs: a national population study. Neurourol Urodyn. 2020;39(3):945-953.
- Jayadevappa R, Chhatre S, Newman DK, et al. Association between overactive bladder treatment and falls among older adults. Neurourol Urodyn. 2018;37(8):2688-2694.
- Gibson W, Hunter KF, Camicioli R, et al. The association between lower urinary tract symptoms and falls: forming a theoretical model for a research agenda. Neurourol Urodyn. 2018;37(1):501-509.
- Noguchi N, Chan L, Cumming RG, et al. Lower urinary tract symptoms and incident falls in community dwelling older men: the Concord Health and Ageing in Men Project. J Urol. 2016;196(6):1694-1699.
- Yoshioka T, Kamitani T, Omae K, et al. Urgency urinary incontinence, loss of independence, and increased mortality in older adults: a cohort study. PLoS One. 2021;16(1):e0245724.
- Kim SJ, Park SG, Pak S, et al. Association between urgency urinary incontinence and cause-specific mortality: a population-based analysis. World J Urol. 2024;43(1):22.
- Peng X, Hu Y, Cai W. Association between urinary incontinence and mortality risk among US adults: a prospective cohort study. BMC Public Health. 2024;24:2753.
- Ha M, Furman A, Al Rihani SB, et al. Pharmacist-driven interventions to de-escalate urinary antimuscarinics in the programs of all-inclusive care for the elderly. J Am Geriatr Soc. 2022;70(11):3230-3238.
- Strong A, Steele E. Optimization of overactive bladder medications in older adults residing in long-term care facilities. Sr Care Pharm. 2025;40:177-184.
- Uren A, Watson M, Dawson S, et al. Identifying the key determinants of a community pharmacy based bladder and bowel service. Res Social Adm Pharm. 2024;20(10):1006-1013.
- Uren A, Dawson S, Cotterill N, et al. The role of community pharmacy in the promotion of continence care: a systematic review. Res Social Adm Pharm. 2024;20(8):689-696.
- University of the West of England Bristol. Pharmacy Role In proMotion of continencE (PRIME). www.uwe.ac.uk/research/centres-and-groups/chcr/research-themes/action/prime. Accessed May 12, 2025.
- Lemanska A, Poole K, Manders R, et al. Patient activation and patient-reported outcomes of men from a community pharmacy lifestyle intervention after prostate cancer treatment. Support Care Cancer. 2022;30(1):347-358.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.