Managing Benign Prostatic Hyperplasia
RELEASE DATE
June 1, 2024
EXPIRATION DATE
June 30, 2026
FACULTY
Donna M. Lisi, PharmD, BCPS, BCGP, BCACP, BCPP, BCMTMS, FASCP
Clinical Pharmacist
Somerset, New Jersey
FACULTY DISCLOSURE STATEMENTS
Dr. Lisi has no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-24-058-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL
To educate pharmacists about the latest American Urological Association guidelines on the pharmacologic management of benign prostatic hyperplasia and lower urinary tract symptoms in men.
OBJECTIVES
After completing this activity, the participant should be able to:
- Describe the relationship between benign prostatic hyperplasia (BPH), benign bladder obstruction, benign prostatic enlargement, and lower urinary tract symptoms (LUTS).
- Define the American Urological Association guideline recommendations on the role of alpha-1 blockers, 5-alpha reductase inhibitors, 5-phosphodiesterase enzyme inhibitors, anticholinergic/antimuscarinic agents, beta-3 agonists, and supplements/nutraceuticals in the management of LUTS attributed to BPH.
- Identify specific concerns regarding the use of pharmacologic classes used in the management of LUTS/BPH.
- Discuss the role that pharmacists can play in optimizing the management of patients with LUTS/BPH.
ABSTRACT: Benign prostatic hyperplasia (BPH) is a histologic diagnosis that refers to the proliferation of smooth muscle, glandular epithelial cells, and connective tissue within the transition zone of the prostate. Benign prostatic obstruction and benign prostatic enlargement can lead to lower urinary tract symptoms (LUTS), but LUTS can form independently of BPH. Common symptoms include nocturia, poor stream, hesitancy, or prolonged micturition. These symptoms can impair a patient's quality of life. Numerous pharmacologic interventions are available, including α-1 blockers, 5-α reductase inhibitors, phosphodiesterase-5 inhibitors, anticholinergic/antimuscarinic agents, and β-3 agonists. Although widely employed, current BPH guidelines do not recommend the use of supplements and nutraceuticals. Interventional, minimally invasive, and surgical options are available for patients with symptoms that are unamenable to pharmacologic interventions. Pharmacists can play a major role in optimizing the drug regimen of patients with LUTS/BPH by conducting thorough medication reviews to eliminate medications that may be exacerbating a patient's symptomatology and by encouraging adherence.
Benign prostatic hyperplasia (BPH), also known as benign prostatic hypertrophy or benign prostatic obstruction, is a histologic diagnosis that refers to the proliferation of smooth muscle, glandular epithelial cells, and connective tissue within the prostatic transition zone (TZ). There are three zones within the prostate: the peripheral zone, the TZ, and the central zone.1-4
FIGURE 1 shows a comparison of a normal and an enlarged prostate.
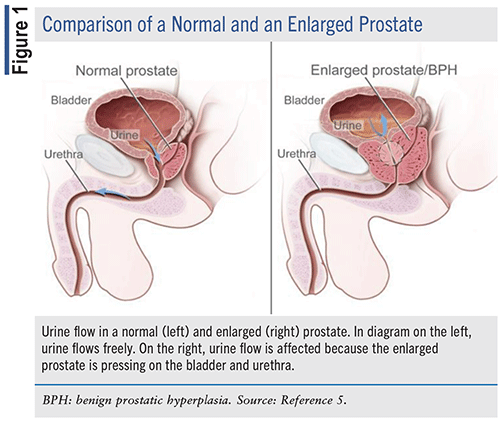
EPIDEMIOLOGY OF BPH
Approximately 14 million men in the United States have BPH.6 According to the American Urological Association (AUA), BPH starts increasing by age 40 to 45 years, with 60% of men having BPH by age 60 years and 80% of men having the condition by age 80 years.2 However, these figures may be underestimations because in the U.S. there are racial disparities in the diagnosis and treatment of BPH.7
PATHOGENESIS OF BPH
The pathogenesis of BPH is multifactorial.4 Among the proposed etiological factors that are associated with the development of BPH are inflammation, changes in the prostatic microbiota, toxic exposures, vitamin D polymorphism, and alterations in telomere length.8-31
Chronic inflammation is considered a key component of benign prostatic obstruction (BPO) in BPH and underlies several of the other proposed mechanisms. Inflammation can be initiated by the presence of infection such as prostatitis, by hormonal alterations, autoimmune disorder, immunological defects, urinary reflux, and even by diet and lifestyle.8-12
PATHOPHYSIOLOGY OF BPH
As BPH refers solely to the histological changes that occur in the prostate, the manifestations of these microscopic changes in the prostate can result in benign prostatic enlargement, or BPE. BPE refers to the increased size of the gland, which usually occurs secondary to BPH. When BPE occurs, it can result in bladder outlet obstruction (BOO), which is defined as the blockage to urinary flow or BPO.4,32,33
BPO and BPE can lead to lower urinary tract symptoms (LUTS), but LUTS can form independently of BPH and may be due to other medical conditions, such as urinary tract infections or overactive bladder (OAB). The term LUTS is used to describe the urinary abnormalities that occur in the bladder and prostate and was previously referred to as prostatism. OAB is a subset of storage LUTS and involves urgency, with or without urgency incontinence, usually with frequency and nocturia. The development of LUTS also increases with age and can occur in parallel to the development of BPH.4,32,33 LUTS in BPH presents as either storage disorders, which manifest as urinary frequency, nocturia, and urinary urgency, or voiding disorders, which involve the presence of a weak or intermittent urine stream, straining to void, hesitancy or inability to start a urine stream, prolonged micturition, or incomplete bladder emptying. The most common LUTS/BPH symptoms are nocturia, poor stream, hesitancy, or prolonged micturition. Prostate enlargement can contribute to the development of LUTS by causing BOO/BPO (static component) or by increasing smooth muscle tone and resistance within the hypertrophied gland (dynamic component). Additionally, OAB resulting from primary detrusor overactivity or underactivity manifests as storage symptoms, but OAB may also result from BPE and BPO secondary to obstruction. It is important to ascertain the cause of LUTS as treatment options vary.4,32,33
BPH DIAGNOSIS AND INTERVENTION
Men typically do not seek treatment until the BPH symptoms become bothersome or severe. These can adversely impact a patient's quality of life (QoL).4
The AUA guidelines recommend that the initial evaluation of BPH symptoms include a thorough medical history (including history of prior urological procedures, sexual history, medication history, overall health and fitness); a physical examination including a digital rectal examination (DRE); a urinalysis to rule out a urinary tract infection and to check for hematuria, proteinuria, and glucosuria; and administering the International Prostate Symptom Score (IPSS).4
The IPSS and the AUA Symptom Index (AUA-SI) are the most widely used primary outcome measures employed in studies evaluating therapies for BPH. Both of these tools consist of seven questions that are used to assess the occurrence over the past month of LUTS associated with BPH. Among the symptoms queried are the presence of incomplete emptying, frequency of urination, hesitancy, urgency, weak stream, straining, and nocturia. Each question is scored on a 0 (not at all) to 5 (almost always) scale and summed to form a final score from 0 to 35. Scoring indicates the severity of symptoms present: mild symptoms (7), moderate symptoms (8-19), and severe symptoms (20-35).34 The IPSS includes both the AUA-SI and an additional question about QoL that is scored on a scale of 0 (delighted) to 6 (terrible) and asks patients how they would feel if they had to live with their urinary condition for the rest of their lives.35
Although the AUA-SI (or IPSS) is a standard metric in drug approval trials, it is a subjective measure. Changes of <3 points on the IPSS are undiscernible, so a minimal, clinically significant difference would have to exceed this number. It is also important to consider baseline symptom scores when evaluating for benefit.36 However, the IPSS has been criticized, as it only weakly correlates with objective voiding parameters.37
A postvoid residual (PVR) volume is used to determine the ability of the bladder to empty and the presence of urinary retention and to evaluate for the presence of detrusor dysfunction. Uroflowmetry is used to assess peak and average flow rates, total void time, and total void volume, with peak urine flow rates <10 mL/sec to 12 mL/sec indicative of obstruction. The healthcare provider should review therapeutic options with the patient, including behavioral/lifestyle modifications, medical therapy, and/or referral to a urologist for surgical interventions.4,36
Upon deciding on a course of therapy, patients should be reevaluated in 4 to 12 weeks (or sooner if adverse events have occurred) to assess the response to the initial interventions. The IPSS should be repeated at this time. If PVR and uroflowmetry have not been performed, they may be considered.4
If a patient remains symptomatic with bothersome LUTS/BPH symptoms despite initial medical treatment or if they experience intolerable adverse events, alternative pharmacologic interventions or referral for surgical interventions should be considered. However, the time frame for follow-up after initiation of treatment is not clear and may depend upon the onset of effects of the drugs employed.4
Additional measurement parameters include prostate volume, return to "normal" symptom severity (i.e., AUA-SI <8), return to normal activities, and sexual function and dysfunction. The International Index of Erectile Dysfuntion (IIEF-EF), specifically the Erectile Function domain (IIEF-5), is the recommended tool to evaluate erectile dysfunction (ED). The minimally clinically important difference is dependent upon baseline erectile function, with improvements of 2 for mild dysfunction, 5 for moderate dysfunction, or 7 for severe ED.36
TREATMENT OF BPH
Lifestyle Modifications
Nonpharmacologic interventions—including behavioral and lifestyle changes; weight loss; exercise; quitting smoking (if applicable); controlling underlying conditions (e.g., diabetes); increasing polyphenols, vitamins, and phytochemicals in the diet; limiting animal protein; and increasing fruits, vegetables, lycopene, and zinc—may hold promise as first steps in reducing the incidence and alleviating symptoms of LUTS/BPH. However, more studies are needed. The AUA has concluded that the use of supplements and nutraceuticals is not warranted.4,38-40
Pharmacologic Management of BPH
If a patient has mild symptoms that are not bothersome, which is generally an AUA-SI (or IPSS) score <7, treatment may not be required and lifestyle changes may be sufficient. If symptoms remain bothersome, pharmacologic interventions should be initiated. In patients with moderate-to-severe symptoms, it is important to determine the patient's goals of care and how treatment may affect their QoL.4
Several drug classes are available for the management of BPH, including α-1 blockers, 5-α reductase inhibitors (5-ARIs), phosphodiesterase-5 inhibitors (PDE5i), β-3 agonists, and anticholinergic (ACh)/antimuscarinic agents.4 TABLE 1 includes the various classes of medications approved for the management of BPH.
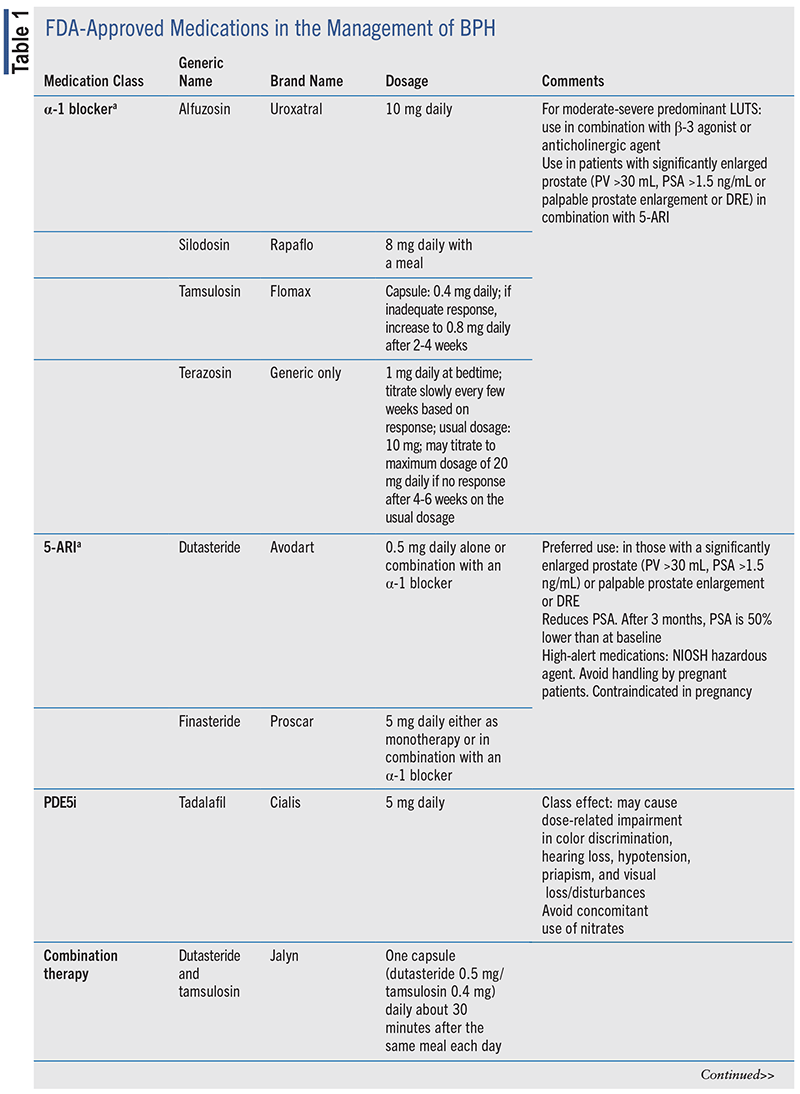
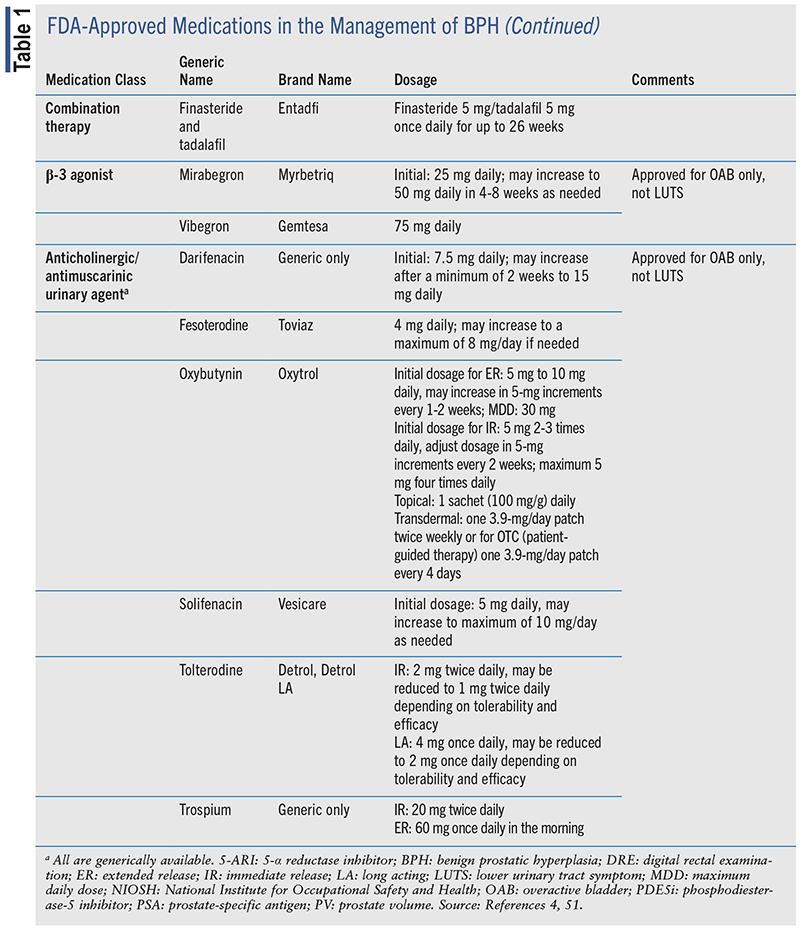
The various therapeutic classes have differing onsets of action in the management of BPH. Among the BPH medications that are fast-acting and can be reevaluated as early as 4 weeks after initiation of therapy are α-1 blockers, β-3 agonists, PDE5i, and ACh/ antimuscarinic agents. The 5-ARIs have a longer onset of action, and it may be advised to wait 3 to 6 months prior to follow-up.4
α-1 Blocker Therapy
The α-1 receptor subfamily is made up of three members: α1A, α-1B, and α-1D. Although all three types of α-1 receptors are found in the genitourinary system, the prostate predominantly expresses the α-1A subtype, which mediates prostate contractions. When the prostate is enlarged, these contractions cause the hyperplastic organ to compress the urethra, resulting in difficulty urinating. α-1B's role in the prostate has not been defined, and α-1D is thought to play a minimal role in inducing contractions.41
First-line therapy for patients with bothersome, moderate-to-severe LUTS secondary to BPH includes α-1 blockers: alfuzosin, doxazosin, tamsulosin, silodosin, and terazosin. In BPH, α-1 antagonism causes smooth muscle relaxation in the bladder neck and prostate via adrenergic blockade, which results in improvement in urine flow rate and reduction of symptoms.42 The different agents in this class appear to be relatively equally effective in improvement of IPSS scores, which is in the range of 4 to 7 points compared with only 2 to 4 points in the placebo group. The choice of an α-blocker depends on patient-specific factors such as age and comorbidities and on a drug's side-effect profile, such as their differing effect on ejaculatory function and blood pressure. It is reasonable that if one α-1 blocker is not effective, changing to a different drug within the class may be beneficial. However, it is not recommended to switch between agents in this class in the presence of adverse effects. No factors have been determined that would predict a more favorable response for one α-blocker over another.4
Tamsulosin was the first α-1A selective agent.41 Other α-1A selective antagonists include silodosin and alfuzosin. On the other hand, terazosin and doxazosin are nonspecific α-1 blockers.4
Class adverse events associated with the α-1 blockers include postural hypotension, which is more common with the nonselective agents, such doxazosin and terazosin, and intraoperative floppy iris syndrome. These agents carry a warning about screening for the presence of prostate cancer prior to initiation and that concomitant use with a PDE5i can result in hypotension.43-47
Floppy iris syndrome is a complication of cataract surgery that is characterized by billowing of a floppy iris stroma (fibrovascular layer of tissue), poor initial mydriasis, sudden loss of pupil dilatation during surgery, and iris prolapse into the corneal incisions.48-50 It can affect patients who are currently on or who were previously on an α-1 antagonist. Discontinuing the α-1 blocker prior to surgery is of no benefit. The ophthalmologist may need to modify the surgical technique used.51
Investigators found that use of α-1 blockers was associated with an increased risk of adverse cardiovascular (CV) outcomes compared with 5-ARIs. Relative risk of harm from α-1 blockers compared with 5-ARIs was 1.08 for 1-year risk of major adverse CV event (MACE; number needed-to-harm [NNH] 160); 1.07 for the composite of MACE and heart failure (NNH 136); and 1.07 for the risk of death (NNH 260). All were statistically significant. However, there was no difference in hospitalizations for heart failure alone. This study raised concern that α-1 blockers may be associated with worse CV outcomes.52 Risk of CV disease with 5-ARIs appears to be less than with α-1 blockers.52
Alfuzosin carries a warning/precaution to use with caution in patients with a history of QT prolongation or who are taking medications that can prolong the QT interval.53,54 It should be discontinued if symptoms of angina pectoris appear or worsen. It is an extended-release formulation, and the tablets should not be chewed or crushed.53 The AUA guidelines on BPH recommend the use of alfuzosin, as it has a low incidence of ejaculatory dysfunction in men.4
Silodosin is considered the most uroselective α1-blocker due to its α1A > α1D > α1B ratio. The advantages of this receptor selectivity include that it may have the greatest effects on obstructive symptoms of LUTS/BPH and has little-to-no CV effects. However, silodosin has the highest rate of ejaculatory dysfunction among the α-1 blockers.55
α-1 blockers can produce ejaculatory dysfunction by causing relaxation of the smooth muscle in the urethra, which results in decreased ejaculatory fluid and may incompletely close the bladder neck, resulting in retrograde ejaculation or anejaculation. More uroselective α-1 blockers are associated with higher rates of ejaculatory dysfunction. The risk of ejaculatory dysfunction appears to be low to slightly increased with terazosin and doxazosin. Tamsulosin is associated with a significant dose-dependent increase in abnormal ejaculation, which may be amenable to every-other-day dosing or a dose reduction if tolerated.56 There is no statistically significant association between the use of α-1 blockers and ED.57
Tamsulosin capsules can be increased to 0.8 mg daily if an inadequate response occurs at the lower dose, but this titration should not occur before 2 to 4 weeks of 0.4-mg dosing. It should not be used with strong cytochrome P450 34A (CYP3A4) inhibitors. Additionally, tamsulosin should be used with caution with moderate CYP3A4 inhibitors, with strong or moderate inhibitors of cytochrome P450 2D6 (CYP2D6) if the patient is known to be a CYP2D6 poor metabolizer, or in combination with other cytochrome P450 inhibitors.58 CYP2D6 polymorphism may affect efficacy of tamsulosin (as measured by IPSS).59 Poor or intermediate CYP2D6 status may also be a risk factor for rare cases of interstitial lung disease associated with tamsulosin use.60 Tamsulosin has been associated with a significant decrease in ejaculatory volume or anejaculation. This could lead to nonadherence.4
Doxazosin and terazosin are nonselective α-1 blockers that are identified as American Geriatrics Society (AGS) Beers Criteria for Potentially Inappropriate Medications (PIMs) in Older Adults because of their high risk of orthostatic hypotension, potential for syncope, and potential to cause urinary incontinence in women.61 When used for BPH, the dose of doxazosin needs to be titrated at 1- to 2-week intervals to a maximum of 8 mg daily.47
Doxazosin fell out of favor following the ALLHAT trial, which concluded that doxazosin increased the risk of heart failure almost twofold in those with diabetes.62,63
Terazosin has a specific warning in its product labeling about the risk of syncope and "first dose" effect in which syncope can occur with the first dose or within a first few days of therapy. Terazosin dosing should be initiated with a 1-mg dose taken at bedtime. The 2-mg, 5-mg, and 10-mg capsules are not indicated for initial dosing. If the drug is discontinued or held for several days, it should be initiated at 1 mg at bedtime. Terazosin labeling also carries a warning about the development of priapism that can lead to permanent impotence in men.64
Tamsulosin has been associated with a 1.85-fold risk of developing delirium compared with nonuse of the α-1 blockers. This risk rose to 2.73-fold with the concomitant use of an ACh/antimuscarinic agent.65
Fall risk is greatest for α-1 blockers during the first month of initiation and during the summer months.66
5-ARI Therapy
5-ARIs, which include finasteride and dutasteride, act by inhibiting the conversion of testosterone to dihydrotestosterone, which is the androgen that is primarily responsible for the normal and abnormal growth of the prostate gland. 5-α reductase (5-AR) exists in two isoforms—type 1 and type 2. Type 1 isoenzyme is responsible for testosterone conversion in the skin and liver, while type 2 isoform regulates testosterone conversion in the prostate. Whereas dutasteride is a competitive and specific inhibitor of both isoforms of 5-AR, finasteride is a competitive and specific inhibitor of 5-AR type 2 isoform.67-69 Dutasteride reduces serum levels of dihydrotestosterone (DHT) by 95%, and finasteride decreases serum levels of DHT by 70%, but it is the reduction produced by the inhibition of isoenzyme type 2 that is most important in BPH.4 Neither dutasteride nor finasteride binds to the androgen receptor.68,69
5-ARIs increase apoptosis within the prostate and can reduce the size of the organ by 15% to 25% following 6 months of treatment. The glandular epithelial component of the prostate is most affected by this enzyme inhibition. This area of the prostate is also the place responsible for the production and release of serum prostate-specific antigen (PSA). As a result, serum PSA and free PSA levels are reduced by about 50%. This may lead to misdiagnosing the presence of prostate cancer if clinicians are not aware that serum PSA levels need to be doubled after 1 year of treatment to adequately assess disease progression.4 Otherwise, the serum PSA would appear low, and the use of serum PSA as a warning sign of prostate cancer may result in missed cases.4 One study found that 7.1% of PSA tests were read as false negatives because the PSA was not adjusted.70
The AUA guidelines recommend 5-ARI monotherapy for symptomatic relief in patients with LUTS/BPH who meet the following criteria: prostate volume >30 cc (on imaging), PSA >1.5 ng/dL, or palpable prostate enlargement on DRE. 5-ARIs can be used either alone or in combination with an α-1 blocker to prevent progression of LUTS/BPH and/or to reduce the risk of acute urinary retention (AUR), which might necessitate future surgery. LUTS/BPH is a progressive disease, and the preemptive use of 5-ARIs may alter the natural course of the disease. Additionally, the larger the prostate gland, the greater is the benefit. 5-ARI therapy has been shown to be more effective than either α-1 blocker monotherapy or placebo in reducing the risk of AUR and surgery for BPH.4
5-ARIs have a slow onset of action, which is an important counseling point. This is due to the drugs' mechanisms of action, which inhibit the growth of the prostate. It takes about 6 months of use to see the full benefit of these drugs.71
5-ARIs improve the IPSS by 3 to 4 points, and this benefit has been observed during 6 to 10 years of follow-up. When finasteride has been compared head-to-head with dutasteride, there were no statistically significant differences in prostate volume, AUA-SI, or Qmax.4
The guidelines advise that prior to initiating 5-ARI therapy, patients should be counseled on the adverse effects of these drugs on sexual function.4 Adverse sexual effects are more common with finasteride.4
An analysis of data from the FDA Adverse Event Reporting System (FAERS) on medications most commonly associated with ED found that 5-ARIs accounted for 46% of ED adverse event reports by medication class between 2010 to 2020.72 In another analysis of FAERS data that examined adverse events associated with 5-ARIs, the investigators found that these events were dose-independent and were more likely to occur in younger patients.73
A condition called postfinasteride syndrome, which has been characterized as a constellation of sexual, physical, and psychological symptoms that include low libido, ED, decreased arousal and difficulty in achieving orgasm, depression, anxiety, and cognitive complaints that remain upon withdrawal, has been reported among men who used finasteride for hair loss.4,74-80
Several studies have identified a higher rate of depression and suicidality among men with LUTS/BPH; however, as in the case of two recent studies, BPH medication use was not studied.81,82 Recently, much attention has been paid to the possible association of the use of 5-ARIs and depression and suicidality. It is known that depressed patients have lower levels of type I 5-AR in the prefrontal cortex, that testosterone and dihydrotestosterone are inversely associated with depression, and that 5-AR is responsible for the production of neuroactive hormones.83
The guidelines also recommend discussing the slightly increased risk of prostate cancer.4 5-ARIs may increase the risk of high-grade prostate cancer as evidenced in the Prostate Prevention Trial, which was the first phase III, randomized, placebo-controlled, prospective, population-based clinical trial to determine if a 5-ARI (finasteride) could prevent the development of prostate cancer.84 The National Cancer Institute has found that there was a 30% reduction in mainly low-grade prostate cancers in men receiving finasteride versus placebo (10.5% vs. 14.9%) after an average of 7 years. They attribute the increase in high-grade cancer in those on finasteride to better detection of the disease rather than being caused by the drug. After 15 years of follow-up, a similar percentage of men (78%) in both groups were still alive.85 However, a study that linked data from the Veterans Affairs Informatics and Computing Infrastructure with the National Death Index to obtain patient records for 80,875 men with American Joint Commission on Cancer stage I to IV prostate cancer who were diagnosed from January 1, 2001, to December 31, 2015, found that the use of 5-ARIs did increase mortality, which was due to a delay in diagnosis of prostate cancer. The median time from first adjusted elevated PSA to diagnosis, mean adjusted PSA at time of biopsy, Gleason grade 8 to 10, higher clinical stage, and incidence of metastatic disease were all significantly increased in the 5-ARI group compared with nonusers. 5-ARI users had significantly higher prostate cancer-specific and all-cause mortality.86 A recent population-based cohort study from Sweden that followed almost 350,000 men for a median follow-up time of 8.2 years found that there was no association between the use of 5-ARIs and the development of prostate cancer. In fact, a time-dependent association was observed with a decreased risk of prostate cancer-related mortality and longer use of 5-ARI therapy.87
According to the prescribing information, finasteride increases the risk of being diagnosed with Gleason score 8 to 10 prostate cancer (finasteride 1.8% vs. placebo 1.1%). Gynecomastia has been reported with these agents.69 Based on findings from the Reduction by Dutasteride of Prostate Cancer Events trial, the 5-ARI also increased the risk of being diagnosed with Gleason score 8 to 10 prostate cancer (dutasteride 1.0% vs. placebo 0.5%).68,88
On June 9, 2011, the FDA issued a safety communication warning about the potential increased risk of a more serious form of prostate cancer from the use of 5-ARIs.89 A systematic review and meta-analysis did not find a statistical difference in death from cancer or death from all causes between men who used 5-ARIs for BPH and those who did not.90
Both finasteride and dutasteride are on the National Institute for Occupational Safety and Health (NIOSH) 2016 list of hazardous medications because of their risk to pregnant women or to women who may potentially be pregnant.91
Sexual dysfunction—which may manifest as ED, ejaculatory dysfunction (i.e., reduced, absent, or painful ejaculation), or hypoactive sexual desire—is significantly increased for 5-ARIs and may be permanent. The odds of ejaculatory dysfunction are over 2.7-fold higher with finasteride compared with placebo and 2.81-fold higher for dutasteride versus placebo. The combination of a 5-ARI and α-1 blocker increases the odds of ejaculatory dysfunction 3.75-fold. The risk appears to be dose dependent, as higher doses of finasteride are associated with ejaculatory dysfunction.56
PDE5i Therapy
There are currently four PDE5i on the market: avanafil, tadalafil, vardenafil, and sildenafil. Of these, only tadalafil is FDA approved for the signs and symptoms of BPH with or without ED.51 If tadalafil is prescribed with finasteride for BPH, it should only be used for up to 26 weeks.46
The mechanism of how tadalafil works in BPH is not clearly understood.4 However, it is hypothesized that tadalafil upregulates nitric oxide/cyclic guanosine monophosphate activity, which decreases smooth muscle tone in the prostatic stroma and capsule and reduces hyperplasia. Other mechanisms have also been proposed including potential anti-inflammatory effects and increasing pelvic perfusion, reducing ischemia, down-regulating Rho-kinase activity, and modulating sympathetic overactivity and afferent nerve activity.92
The effect of tadalafil on IPSS scores compared with placebo is unclear.4 Tadalafil and sildenafil may help relieve nocturia associated with BPH.93,94 The AUA guidelines recommend the use of tadalafil as a reasonable option in selected men with BPH who have ED.4
If avanafil is used with an α-1 blocker, the dose should be decreased from 100 mg to 50 mg.43 Sildenafil should be initiated at 25 mg if it is coadministered with an α-1 blocker instead of the initial dose of 50 mg.44 Vardenafil's prescribing information states that for patients on stable α-1 blocker therapy who are started on the PDE5i, the dose should be 5 mg, which is one-half of the normal starting dose.45 According to the prescribing information, it is not recommended to give tadalafil with an α-1 blocker in the treatment of BPH because the efficacy of the combination has not been adequately studied and because of the risk of hypotension. If tadalafil is being used in a patient with ED who is taking an α-1 blocker, caution is advised.46
5-ARI and α-1 Blocker
Combination Therapy
The AUA guidelines recommend that combination therapy with a 5-ARI and α-1 blocker should be offered as a treatment option for LUTS only to men with BPH with a prostate volume >30 cc (on imaging), PSA >1.5 ng/dL, or palpable prostate on DRE.4,95,96
PDE5i and α-1 Blocker or 5-ARI
Combination Therapy
In a 2023 amendment to the 2021 guidelines, the AUA expanded its recommendations to include the use of low-dose daily tadalafil 5 mg with the use of an α-1 blocker for the treatment of LUTS/BPH.2 Previously, the AUA guidelines published in 2021 had advised against the use of combination low-dose daily tadalafil 5 mg with α-1 blockers for the treatment of LUTS/BPH because it was felt that coadministration did not offer advantages in symptom improvement compared with the use of either drug alone.4 The reference cited in the guidelines for this recommendation was a study in which tadalafil 5 mg, silodosin 8 mg, or tadalafil 5 mg/silodosin 8 mg daily for 3 months were studied in men with LUTS related to BPH. There was significant improvement in Qmax, IPSS scores, PVR, and IIEF scores with the combination of tadalafil and silodosin compared with either drug alone.97 However, despite the statistical difference, the AUA guideline authors felt that there was little-to-no clinical difference in mean IPSS scores between the groups.2 The combination regimen appears to be beneficial in reducing voiding symptoms.98 There are concerns about hypotension with the concomitant administration of an α-1 blocker and a PDE5i.4
Tadalafil has been shown to be associated with a decreased risk of MACE/venous thromboembolism in men with LUTS compared with men who were either receiving or not receiving an α-1 blocker.99 This finding may be encouraging, as recently it was observed that α-1 blocker use was associated with an increased risk of adverse CV outcomes, including 1-year risk of MACE, a composite of MACE and heart failure, and the risk of death in men with BPH.52
The 2023 AUA guideline amendment also indicates that the combination of low-dose daily tadalafil 5 mg with finasteride may be tried in patients with LUTS/ BPH.2 Dutasteride as add-on therapy was found to be beneficial in men with BPH who did not receive adequate relief of LUTS with tadalafil.100 However, long-term data are needed for these combination regimens.2
In December 2021, a combination product of daily fixed-dose finasteride 5 mg and tadalafil 5 mg was approved by the FDA as initial treatment for signs and symptoms (up to 26 weeks) in men with BPH. This combination product has been considered the "commercialization of the prescribing cascade" because in those with baseline ED or those who develop ED while on finasteride, the 5-ARI should be discontinued. This combination represents an avoidable prescribing cascade. Concern has been raised that this product may serve a niche market of sexually active younger men with LUTS and enlarged prostates who may be at an increased risk for suicidality and depression associated with finasteride use.101
ACh Agents
Darifenacin, fesoterodine, oxybutynin, solifenacin, tolterodine, and trospium are ACh/antimuscarinic agents that are FDA approved for use in OAB, but none are approved for use in BPH or LUTS/BPH. The AUA guidelines state that ACh agents with or without an α-1 blocker may be considered as a treatment option for patients with moderate-to-severe predominant storage LUTS. It is important that a PVR be obtained before initiation of therapy and that these agents be used in patients with urodynamically proven obstruction and OAB. Studies involving combination therapy with an α-1 blocker and ACh agent have been of short duration, typically 12 weeks, with variable effects on IPSS scores.4
The 2023 amendment to the AUA guidelines added additional clarification for the use of ACh/antimuscarinic agents in the management of LUTS/BPH. It stated that these agents alone or combination with an α-1 blocker may be offered to patients with moderate-to-severe predominant storage LUTS. They also added a warning to avoid the use of these agents in patients with gastric emptying/gastric motility issues and in narrow-angle glaucoma. The guidelines also caution that the use of ACh/antimuscarinic agents has been associated with an increased risk of dementia in people aged older than 55 years and most especially in people aged older than 70 years.2
While urinary urgency and incontinence are themselves fall risks, ACh/antimuscarinic agents can further exacerbate this risk by producing dizziness, somnolence, visual impairment, and orthostasis.102
The 2023 AUA guidelines amendment also elaborates on the combination use of ACh/antimuscarinic agents and α-1 blockers. While the combination regimen may seem justified in storage-dependent LUTS/BPH, the authors caution that the impact on IPSS scores is variable compared with α-1 blocker monotherapy and that these drugs are associated with increased adverse effects. More studies are needed to determine the combination regimen's place in therapy.2
The 2023 AGS Beers Criteria for PIMs in Older Adults recommends avoiding the use of strongly ACh drugs, except antimuscarinics for urinary incontinence in men with BPH. Use of these highly ACh medications may decrease urinary flow and cause urinary retention. This is a strong recommendation that is supported by moderate-quality evidence.61
It is important to recognize ACh adverse events, especially since many men with LUTS/BPH are older.103 It is best to use the lowest dose of a urinary antimuscarinic agent as possible to minimize ACh burden.104
A systematic review and network meta-analysis compared the combination regimens of an α-1 blocker and mirabegron (a β-3 agonist) and an α-1 blocker and ACh/antimuscarinic agent in men with LUTS secondary to BPH and OAB. Studies were parallel-group, randomized clinical trials that lasted >8 weeks. There were no statistically significant differences in safety or efficacy analyses (i.e., the primary outcomes, which were micturition/day, incontinence episodes/day, and urgency episodes/day or the secondary efficacy outcomes of a score for OAB and the IPSS), although nonsignificant numerical differences did exist between the groups.105
β-3 Agonists
While the two FDA-approved β-3 agonists mirabegron and vibegron are approved for OAB, they are not approved for BPH or LUTS/BPH.
The AUA guidelines caution that monotherapy with a β-3 agonist has not been shown to significantly improve LUTS/BPH symptoms but that in combination with an α-1 blocker, improvement in symptoms might occur that is comparable to that seen with the use of ACh/antimuscarinic agents.4
The 2023 amendment to the AUA guidelines further delineates that these drugs may be offered in combination with an α-1 blocker for men with moderate-to-severe, storage-predominant LUTS.2 Treatment with a mirabegron 50-mg plus doxazosin 2-mg regimen was superior to the tolterodine 4-mg plus doxazosin 2-mg regimen in improving erectile function, but there was no difference between the regimens' IPSS scores, QoL, and PVR after 4 or 12 weeks. Although adverse events were numerically less common in the mirabegron group (vs. the tolterodine group), this did not reach statistical significance.106
The amended guidelines recommend that the use of β-3 agonists may be a suitable option in older adults for whom the decision is made to avoid ACh/antimuscarinic agents.2
Additionally, an updated systematic review and meta-analysis examining the role of antimuscarinics combined with α-1 blockers in the management of urinary storage symptoms in patients with BPH concluded that adding an ACh agent to an α-1 blocker did not result in a significant decrease in urgency and micturition episodes and that the toxicity profile does not favor antimuscarinic use.107
Complementary and Alternative Medicine
The AUA guidelines do not address the use of complementary and alternative medicine, dietary supplements, or nutraceuticals in detail. They cite methodological issues and shortcomings that cast doubt on the findings of positive clinical trials.4
NONPHARMACOLOGIC INTERVENTIONS
Interventional procedures (i.e., botulinum toxin A [onabotulinumtoxin A], acupuncture or electroacupuncture with/without moxibustion, pulmonary artery embolization), minimally invasive procedures, or surgery (transurethral resection of the prostate or open prostatectomy) may be necessary when oral medications that are intended to relieve the symptoms of BPH fail.2,4 These topics are beyond the scope of this article.
THE PHARMACIST'S ROLE
There is a paucity of information on the role of the pharmacist in managing patients with BPH. However, pharmacists can have a major impact in these patients' care by optimizing their drug regimens and conducting thorough medication histories to identify drugs that may be exacerbating BPH symptoms.108
It is important to review a patient's medical history and determine the onset and duration of symptoms and whether their appearance is related to the initiation of a new medication that can adversely affect their prostate health; if they are using any other dietary supplements or OTC medications to manage their symptoms or if they have used any of these in the past; and the severity of symptoms and how they are impacting the patient's QoL. Asking about sexual history and reviewing a patient's medication list for the presence of any medications that can adversely affect sexual function are also important.
Pharmacist involvement may impact BPH symptomatology and QoL among those with BPH. A longitudinal retrospective study involved 102 men aged 50 to 80 years (mean age: 69.9 years) with BPH who were enrolled in Johns Hopkins Aramco Healthcare in Saudi Arabia assessed PSA values over a 2-year period that examined the effect of severity of BPH symptoms on patients' QoL and the effectiveness of pharmacist interventions in improving symptom scores as measured by the IPSS and QoL. For patients with moderate-to-severe IPSS scores, pharmacists' recommendations resulted in the addition of the following drugs to the patients' regimens: doxazosin, mirabegron, mirabegron combinations (+ solifenacin or + oxybutynin), and solifenacin. QoL scores improved from baseline following the pharmacist's interventions. However, shortcomings of this trial limit its utility.109
Nonadherence is another medication-related problem seen in the management of BPH.110,111 One study found that adherence to BPH medications at 6 month follow-up was 32% and fell to 23% at 12 months. There was a direct correlation between the degree of LUTS/BPH symptomatology and adherence.110 Another study found that only 48% of patients with LUTS/BPH were still on treatment after ≥4 years. Those who were prescribed combination therapy with an α-1 blocker and 5-ARI were most adherent, with 63.5% still on treatment at ≥4 years compared with those on 5-ARI monotherapy who were least adherent at 29.2%.111
A study conducted in Jordan found that compared with usual care offered by a urologist, the patients in the clinical pharmacist group were significantly more adherent to treatment and had an improved QoL.112
Pharmacists need to educate patients about the dangers of ordering pharmaceuticals for BPH over the Internet. Researchers found that advertisements for BPH-related products were easily retrievable from search engines and had better readability than nonadvertisements, but they were more often biased and provided the reader with lower quality information.113
CONCLUSION
Pharmacists need to be familiar with the various medication classes and drugs within those classes that are indicated for the management of LUTS/BPH. With the plethora of medication options available for the management of symptoms of LUTS/BPH and the specific indications for their use, pharmacists can help with appropriate drug selection, screening drug regimens for medications that may exacerbate or may be contributing to symptoms, monitoring for efficacy and adverse events, and promoting and monitoring patient adherence. When appropriate, pharmacists should also be able to recognize when it is important to refer the patient to a urologist for further evaluation.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. National Institute of Diabetes and Digestive and Kidney Disorders.
Prostate enlargement (benign prostatic hyperplasia). www.niddk.nih.gov/health-information/urologic-diseases/prostate-problems/prostate-enlargement-benign-prostatic-hyperplasia. Accessed May 1, 2024.
2. Sandhu JS, Bixler BR, Dahm P, et al. Management of lower urinary
tract symptoms attributed to benign prostatic hyperplasia (BPH): AUA
Guideline amendment 2023. J Urol. 2024;211(1):11-19.
3. Ali A, Du Feu A, Oliveira P, et al. Prostate zones and cancer: lost in
transition? Nat Rev Urol. 2022;19(2):101-115.
4. Lerner LB, McVary KT, Barry MJ, et al. Management of lower urinary
tract symptoms attributed to benign prostatic hyperplasia: AUA Guideline
part I—initial work-up and medical management. J Urol. 2021;206(4):806-817.
5. National Cancer Institute. Understanding prostate changes and conditions. www.cancer.gov/types/prostate/understanding-prostate-changes.
Accessed May 1, 2024.
6. Deters LA. Benign prostatic hyperplasia (BPH). Medscape. May 22,
2023. https://emedicine.medscape.com/article/437359-overview#a5. Accessed
May 1, 2024.
7. Ayangbesan A, Kavoussi N. Racial disparities in diagnosis and management of benign prostatic hyperplasia: a review. Curr Urol Rep.
2022;23(11):297-302.
8. Gacci M, Nunzio C, Gravas S. Unveiling prostatic inflammation to
optimize management of lower urinary tract symptoms: a discussion with
experts. Biomark Med. 2023;17(18):739-745.
9. Tsunemori H, Sugimoto M. Effects of inflammatory prostatitis on the
development and progression of benign prostatic hyperplasia: a literature
review. Int J Urol. 2021;28(11):1086-1092.
10. Horsanali MO, Dil E, Caglayan A, et al. The predictive value of the
systemic immune-inflammation index for the progression of lower urinary
tract symptoms in men. Asian Pac J Cancer Prev. 2023;24(11):3845-3850.
11. Arora B, Khan M, Pridgeon S. Does histological prostatic inflammation during transurethral resection of the prostate for bladder outlet
obstruction affect post-operative urinary outcomes? Low Urin Tract
Symptoms. 2023;15(2):57-62.
12. Tang G, Liu M, Ding G, et al. The efficacy of cyclooxygenase-2
inhibitors for the male treatment of lower urinary tract symptoms: a
systematic review and meta-analysis. Am J Mens Health.
2023;17(3):15579883231176667.
13. Li J, Li Y, Zhou L, et al. The human microbiome and benign prostatic
hyperplasia: current understandings and clinical implications. Microbiol
Res. 2024;281:127596.
14. Russo GI, Bongiorno D, Bonomo C, et al. The relationship between
the gut microbiota, benign prostatic hyperplasia, and erectile dysfunction.
Int J Impot Res. 2023;35(4):350-355.
15. Li J, Li Y, Zhou L, et al. Microbiome analysis reveals the inducing
effect of Pseudomonas on prostatic hyperplasia via activating NF-κB
signalling. Virulence. 2024;15(1):2313410.
16. Mjaess G, Karam A, Roumeguère T, et al. Urinary microbiota and
prostatic diseases: the key for the lock? A systematic review. Prostate
Cancer Prostatic Dis. 2023;26(3):451-460.
17. Suarez Arbelaez MC, Monshine J, Porto JG, et al. The emerging role
of the urinary microbiome in benign noninfectious urological conditions:
an up-to-date systematic review. World J Urol. 2023;41(11):2933-2948.
18. Xia D, Wang J, Zhao X, et al. Association between gut microbiota
and benign prostatic hyperplasia: a two-sample Mendelian randomization
study. Front Cell Infect Microbiol. 2023;13:1248381.
19. Mariotti ACH, Heidrich V, Inoue LT, et al. Urinary microbiota is
associated to clinicopathological features in benign prostatic hyperplasia.
Prostate. 2024;84(3):285-291.
20. Takezawa K, Fujita K, Matsushita M, et al. The Firmicutes/Bacteroidetes ratio of the human gut microbiota is associated with prostate enlargement. Prostate. 2021;81(16):1287-1293.
21. Liao G, Lee PMY, Zhao S, et al. Joint effect between bisphenol A and
alcohol consumption on benign prostatic hyperplasia: a case-control study
in Hong Kong Chinese males. Prostate. 2021;81(15):1214-1224.
22. Washington State Department of Health. BPA and phthalates. March
2014. https://doh.wa.gov/sites/default/files/legacy/Documents/Pubs//210-090E_BPAandPhthalates.pdf. Accessed May 7, 2024.
23. Yang L, Liu Z, Peng Z, et al. Exposure to Di-2-ethylhexyl phthalate
and benign prostatic hyperplasia, NHANES 2001-2008. Front Endocrinol
(Lausanne). 2022;12:804457.
24. Singh V, Madeshiya AK, Ansari NG, et al. CYP1A1 gene polymorphism
and heavy metal analyses in benign prostatic hyperplasia and prostate
cancer: an explorative case-control study. Urol Oncol. 2023;41(8):355.
e9-355.e17.
25. Ruan L. Association between vitamin D receptor gene polymorphisms
and genetic susceptibility to benign prostatic hyperplasia: a systematic
review and meta-analysis. Medicine (Baltimore). 2024;103(9):e37361.
26. Lin L, Li P, Liu X, et al. Systematic review and meta-analysis of candidate gene association studies of benign prostate hyperplasia. Syst
Rev. 2022;11(1):60.
27. Ermec B, Culha MG, Kocak G, et al. The effect of vitamin D replacement in patients with lower urinary tract complaint/erectile dysfunction
resistant to tadalafil 5 mg treatment: a pilot clinical study. Andrologia.
2022;54(8):e14473.
28. National Human Genome Research Institute. Telomere. May 7, 2024.
www.genome.gov/genetics-glossary/Telomere. Accessed May 7, 2024.
29. Arbeev KG, Verhulst S, Steenstrup T, et al. Association of leukocyte
telomere length with mortality among adult participants in 3 longitudinal
studies. JAMA Netw Open. 20205;3(2):e200023.
30. Tu L, Huda N, Grimes BR, et al. Widespread telomere instability in
prostatic lesions. Mol Carcinog. 2016;55(5):842-852.
31. Lv K, Wu Y, Yang G, et al. Leukocyte telomere length and the risk of
prostate cancer and benign prostatic hyperplasia: insights from UK Biobank
and Mendelian Randomization Study. J Gerontol A Biol Sci Med Sci.
2024;79(3):glad272.
32. Kim MK, Shin YS, Lee JH, et al. The prevalence of lower urinary tract
symptoms and overactive bladder in South Korea: a cross-sectional,
population-based study. Int Neurourol J. 2022;26(1):31-36.
33. Ng M, Leslie SW, Baradhi KM. Benign prostatic hyperplasia. In:
StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024
Jan-.
34. Barry MJ, Fowler FJ Jr, O'Leary MP, et al. The American Urological
Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol.
1992;148:1549.
35. Electronic Clinical Quality Measures. International Prostate Symptom
Score (IPSS) or the American Urological Association Symptom Index
(AUA-SI) change 6-12 months after diagnosis of benign prostatic hyperplasia. https://ecqi.healthit.gov/sites/default/files/ecqm/measures/CMS771v1.html#:~:text=The%20IPSS%20is%20inclusive%20of,are%20the%20urinary%20symptom%20score. Accessed May 7, 2024.
36. FDA. Non-clinical and clinical investigation of devices used for the
treatment of benign prostatic hyperplasia (BPH). Guidance for industry
and Food and Drug Administration staff. December 27, 2021. www.fda.gov/media/79397/download. Accessed May 7, 2024.
37. Kohler TS, Kausik SJ. Comparison of IPSS score and voiding parameters in men presenting with LUTS. Can J Urol. 2023;30(5):11668-11675.
38. Stewart KL, Lephart ED. Overview of BPH: symptom relief with
dietary polyphenols, vitamins and phytochemicals by nutraceutical supplements with implications to the prostate microbiome. Int J Mol Sci.
2023;24(6):5486.
39. Mousavi SN, Nouri M, Yousefi Rad E, et al. Association between
dietary phytochemical index and risk of benign prostatic hyperplasia: a
case-control study. J Health Popul Nutr. 2024;43(1):35.
40. Cicero AFG, Allkanjari O, Busetto GM, et al. Nutraceutical treatment
and prevention of benign prostatic hyperplasia and prostate cancer. Arch
Ital Urol Androl. 2019;91(3).
41. Akinaga J, García-Sáinz JA, Pupo AS. Updates in the function and
regulation of α1-adrenoceptors. Br J Pharmacol. 2019;176(14):2343-2357.
42. Deters LA. Benign prostate hyperplasia (BPH) medication. Medscape.
March 22, 2023. https://emedicine.medscape.com/article/437359-medication#:~:text=Alfuzosin%20is%20indicated%20for%20the,reduction%20in%20symptoms%20of%20BPH. Accessed May 7,
2024.
43. Stendra (avanafil) product information. Freehold, NJ: Metuchen
Pharmaceuticals; October 2022.
44. Viagra (sildenafil citrate nitrate) product information. New York, NY:
Pfizer Laboratories; December 2017.
45. Vardenafil hydrochloride product information. Congers, NY: Chartwell
Rx; February 2024.
46. Cialis (tadalafil) product information. Indianapolis, IN: Eli Lilly USA;
April 2023.
47. Cardura (doxazosin mesylate) product information. New York, NY:
Roerig/Pfizer; January 2022.
48. Maluskova M, Vidlar A, Maresova K, et al. Floppy iris syndrome
associated with specific medication intake: a narrative review. Biomed Pap
Med Fac Univ Palacky Olomouc Czech Repub. 2023;167(1):9-15.
49. González Martín-Moro J, Muñoz Negrete F, Lozano Escobar I,
Fernández Miguel Y. Intraoperative floppy-iris syndrome. Arch Soc Esp
Oftalmol. 2013;88(2):64-76.
50. Kumar A, Raj A. Intraoperative floppy iris syndrome: an updated
review of literature. Int Ophthalmol. 2021;41(10):3539-3546.
51. Lexicomp Online. Waltham, MA: UpToDate, Inc; 2023. https://online.lexi.com. Accessed February 1, 2024.
52. Zhang J, Latour CD, Olawore O, et al. Cardiovascular outcomes of
α-blockers vs 5-α reductase inhibitors for benign prostatic hyperplasia.
JAMA Netw Open. 2023;6(11):e2343299.
53. Uroxatral (alfuzosin hydrochloride, extended release) product information. Dublin, Ireland: Concordia Pharmaceuticals/Amdipharm Limited; May 2020.
54. Lepor H, Lepor NE, Hill LA, Trohman RG. The QT interval and
selection of alpha-blockers for benign prostatic hyperplasia. Rev Urol.
2008;10(2):85-91.
55. Califano G, Collà Ruvolo C, Creta M, et al. Focus on silodosin: pros
and cons of uroselectivity. Res Rep Urol. 2020;12:669-672.
56. Bearelly P, Avellino GJ. The role of benign prostatic hyperplasia treatments in ejaculatory dysfunction. Fertil Steril. 2021;116(3):611-617.
57. Bapir R, Bhatti KH, Eliwa A, et al. Effect of alpha-adrenoceptor
antagonists on sexual function. A systematic review and meta-analysis.
Arch Ital Urol Androl. 2022;94(2):252-263.
58. Flomax (tamsulosin hydrochloride) product information. Bridgewater,
NJ: Sanofi-Aventis US LLC; January 2019.
59. Abdullaev SP, Shatokhin MN, Tuchkova SN, et al. Effects of CYP2D6
allelic variants on therapy with tamsulosin in patients with benign prostatic
hyperplasia. Drug Metab Pers Ther. 2023;38(4):323-330.
60. Jessurun NT, Wijnen PA, Bast A, et al. Tamsulosin associated with
interstitial lung damage in CYP2D6 variant alleles carriers. Int J Mol Sci.
2020;21(8):2770.
61. 2023 American Geriatrics Society Beers Criteria Update Expert Panel.
American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc.
2023;71(7):2052-2081.
62. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major cardiovascular events in hypertensive
patients randomized to doxazosin vs chlorthalidone: the Antihypertensive
and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT).
JAMA. 2000;283(15):1967-1975.
63. Barzilay JI, Davis BR, Bettencourt J, et al. Cardiovascular outcomes
using doxazosin vs. chlorthalidone for the treatment of hypertension in
older adults with and without glucose disorders: a report from the ALLHAT
study. J Clin Hypertens (Greenwich). 2004;6(3):116-125.
64. Terazosin product information. Congers, NY: Chartwell Rx; February
2023.
65. Kotake K, Noritake Y, Kawakami Y. Association of silodosin, tamsulosin, and naftopidil with delirium: analysis of the pharmacovigilance
database in Japan. Int J Clin Pharm. 2023;45(5):1252-1259.
66. Seo JH, Han JS, Lee Y, et al. Fall risk related to subtype-specific alpha-antagonists for benign prostatic hyperplasia: a nationwide Korean population-based cohort study. World J Urol. 2022;40(12):3043-3048.
67. Marks LS. 5-alpha-reductase: history and clinical importance. Rev
Urol. 2004;6(Suppl 9):S11-S21.
68. Avodart (dutasteride) product information. Wixom, MI: Waylis
Therapeutics, Inc; October 2023.
69. Proscar (finasteride) product information. Jersey City, NJ: Organon
LLC; November 2023.
70. Loloi J, Wei M, Babar M, et al. Rates of false-negative screening in
prostate specific antigen secondary to 5-alpha reductase inhibitor usage:
a quality-improvement initiative. Int Braz J Urol. 2022;48(4):688-695.
71. WebMD. What to know about 5-alpha reductase inhibitors. July 18,
2023. www.webmd.com/men/prostate-enlargement-bph/what-to-know-5-alpha-reductase-inhibitors. Accessed May 7, 2024.
72. Kaplan-Marans E, Sandozi A, Martinez M, et al. Medications most
commonly associated with erectile dysfunction: evaluation of the Food
and Drug Administration National Pharmacovigilance Database. Sex Med.
2022;10(5):100543.
73. Harrell MB, Ho K, Te AE, et al. An evaluation of the federal adverse
events reporting system data on adverse effects of 5-alpha reductase
inhibitors. World J Urol. 2021;39(4):1233-1239.
74. Diviccaro S, Melcangi RC, Giatti S. Post-finasteride syndrome: an
emerging clinical problem. Neurobiol Stress. 2019;12:100209.
75. Traish AM. Post-finasteride syndrome: a surmountable challenge for
clinicians. Fertil Steril. 2020;113(1):21-50.
76. Romero Pérez P. Post-finasteride syndrome. Literature review. Arch
Esp Urol. 2022;75(5):382-399.
77. Zhang JJ, Shi X, Wu T, et al. Sexual, physical, and overall adverse
effects in patients treated with 5α-reductase inhibitors: a systematic review
and meta-analysis. Asian J Androl. 2022;24(4):390-397.
78. Public Citizen. Post-Finasteride Syndrome Foundation v. FDA. www.citizen.org/litigation/post-finasteride-syndrome-foundation-v-fda/. Accessed
May 7, 2024.
79. Low P, Li KD, Hakam N, et al. 5-alpha reductase inhibitor related
litigation: a legal database review. Andrology. 2022;10(3):470-476.
80. Nguyen DD, Fellouah M, Nguyen AV, et al. Litigation associated with
5-alpha-reductase-inhibitor use: a Canadian legal database review. Can J
Urol. 2023;30(3):11546-11550.
81. Zhang W, Cao G, Sun Y, et al. Depressive symptoms in individuals
diagnosed with lower urinary tract symptoms suggestive of benign prostatic
hyperplasia (LUTS/BPH) in middle-aged and older Chinese individuals:
results from the China Health and Retirement Longitudinal Study. J Affect
Disord. 2022;296:660-666.
82. Lee SU, Lee SH, So AH, et al. Association between benign prostatic
hyperplasia and suicide in South Korea: a nationwide retrospective cohort
study. PLoS One. 2022;17(3):e0265060.
83. Welk B, McArthur E, Ordon M, et al. Association of suicidality and
depression with 5α-reductase inhibitors. JAMA Intern Med. 2017;177(5):683-
691.
84. Thompson IM, Tangen C, Goodman P. The Prostate Cancer Prevention
Trial: design, status, and promise. World J Urol. 2003;21(1):28-30.
85. National Cancer Institute. Prostate Cancer Prevention Trial (PCPT):
questions and answers. July 10, 2023. www.cancer.gov/types/prostate/research/prostate-cancer-prevention-trial-qa. Accessed May 7, 2024.
86. Sarkar RR, Parsons JK, Bryant AK, et al. Association of treatment
with 5α-reductase inhibitors with time to diagnosis and mortality in
prostate cancer. JAMA Intern Med. 2019;179(6):812-819.
87. Björnebo L, Nordström T, Discacciati A, et al. Association of 5α-reductase
inhibitors with prostate cancer mortality. JAMA Oncol. 2022;8(7):1019-
1026.
88. Andriole GL, Bostwick DG, Brawley OW, et al. Effect of dutasteride
on the risk of prostate cancer. N Engl J Med. 2010;362(13):1192-1202.
89. FDA. 5-alpha reductase inhibitors (5-ARIs) may increase the risk of
a more serious form of prostate cancer. February 8, 2018. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-5-alpha-reductase-inhibitors-5-aris-may-increase-risk-more-serious. Accessed May
7, 2024.
90. Park JJ, Lee HY, Shim SR, et al. Prostate cancer specific mortality after
5α-reductase inhibitors medication in benign prostatic hyperplasia patients:
systematic review and meta-analysis. Aging Male. 2021;24(1):80-91.
91. Connor TH, MacKenzie BA, DeBord DG, et al. NIOSH list of antineoplastic and other hazardous drugs in healthcare settings, 2016. National
Institute for Occupational Safety and Health. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and
Prevention, National Institute for Occupational Safety and Health, DHHS
(NIOSH) Publication Number 2016-161 (Supersedes 2014-138). www.cdc.gov/niosh/docs/2016-161/pdfs/2016-161.pdf. Accessed May 16, 2024.
92. Hatzimouratidis K. A review of the use of tadalafil in the treatment
of benign prostatic hyperplasia in men with and without erectile dysfunction. Ther Adv Urol. 2014;6(4):135-147.
93. Ko WJ, Han HH, Ham WS, Lee HW. Daily use of sildenafil 50mg at
night effectively ameliorates nocturia in patients with lower urinary tract
symptoms associated with benign prostatic hyperplasia: an exploratory
multicenter, double-blind, randomized, placebo-controlled study. Aging
Male. 2017;20(2):81-88.
94. Kuno T, Tamura K, Fukuhara H, et al. Tadalafil 5 mg once daily
improved each IPSS subscore, QOL, and nocturia in elderly BPH patients
over 70 years old in a real-world clinical setting. Urol Int. 2022;106(10):1005-
1011.
95. Roehrborn CG, Siami P, Barkin J, et al. The effects of dutasteride,
tamsulosin and combination therapy on lower urinary tract symptoms in
men with benign prostatic hyperplasia and prostatic enlargement: 2-year
results from the CombAT study. J Urol. 2008;179(2):616-621.
96. McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term
effect of doxazosin, finasteride, and combination therapy on the clinical
progression of benign prostatic hyperplasia. N Engl J Med. 2003;349(25):2387-
2398.
97. Kuno T, Tamura K, Fukuhara H, et al. Tadalafil 5 mg once daily
improved each IPSS subscore, QOL, and nocturia in elderly BPH patients
over 70 years old in a real-world clinical setting. Urol Int. 2022;106(10):1005-
1011.
98. AbdelRazek M, Abolyosr A, Mhammed O, et al. Prospective comparison of tadalafil 5 mg alone, silodosin 8 mg alone, and the combination
of both in treatment of lower urinary tract symptoms related to benign
prostatic hyperplasia. World J Urol. 2022;40(8):2063-2070.
99. Goberdhan S, Blachman-Braun R, Nackeeran S, et al. Is tadalafil
associated with decreased risk of major adverse cardiac events or venous
thromboembolism in men with lower urinary tract symptoms? World J
Urol. 2022;40(7):1799-1803.
100. Wada N, Abe N, Miyauchi K, et al. Dutasteride add-on treatment
to tadalafil for patients with benign prostatic enlargement is similarly
effective as dutasteride add-on treatment to alpha blocker: a propensity-
score matching analysis. Int Urol Nephrol. 2022;54(6):1193-1198.
101. Nguyen DD, Trinh QD, Bhojani N. Combination of tadalafil and
finasteride for the treatment of urinary tract symptoms related to benign
prostatic hyperplasia: commercialization of the prescribing cascade. Eur
Urol. 2022;81(4):323-324.
102. İlhan B, Erdoğan T, Topinková E, et al. Management of use of urinary
antimuscarinics and alpha blockers for benign prostatic hyperplasia in
older adults at risk of falls: a clinical review. Eur Geriatr Med. 2023;14(4):733-
746.
103. Lisi DM. Assessing anticholinergic effects in older adults. In Amornyotin S, ed. Update in Geriatrics. London, England: IntechOpen; 2021.
104. Kim TH, Jung W, Suh YS, et al. Comparison of the efficacy and safety of tolterodine 2 mg and 4 mg combined with an α-blocker in men
with lower urinary tract symptoms (LUTS) and overactive bladder: a
randomized controlled trial. BJU Int. 2016;117(2):307-315.
105. Herschorn S, Tarcan T, Jiang YH, et al. Safety and efficacy of an
α1-blocker plus mirabegron compared with an α1-blocker plus antimuscarinic in men with lower urinary tract symptoms secondary to benign
prostatic hyperplasia and overactive bladder: a systematic review and
network meta-analysis. Neurourol Urodyn. 2024;43(3):604-619.
106. Elbaz R, El-Assmy A, Zahran MH, et al. Mirabegron for treatment
of erectile dysfunction concomitant with lower urinary tract symptoms in
patients with benign prostatic obstruction: a randomized controlled trial.
Int J Urol. 2022;29(5):390-396.
107. Lenfant L, Pinar U, Roupret M, et al. Role of antimuscarinics combined with alpha-blockers in the management of urinary storage symptoms
in patients with benign prostatic hyperplasia: an updated systematic review
and meta-analysis. J Urol. 2023;210(1):34-35.
108. Renoncourt T, Saint F, Bennis Y, et al. Potentially inappropriate
prescribing for prostatic hyperplasia in older persons. J Am Med Dir Assoc.
2022;23(6):992-997.
109. Al Makahleh B, Sattar AHT, Thorakkattil SA, et al. Pharmacist-led
benign prostatic hyperplasia medication management to an optimal goal
in the ambulatory care settings: a longitudinal study. Saudi J Clin Pharm.
2023;2:125-130.
110. Zabkowski T, Saracyn M. Drug adherence and drug-related problems
in pharmacotherapy for lower urinary tract symptoms related to benign
prostatic hyperplasia. J Physiol Pharmacol. 2018;69(4).
111. Shortridge E, Donatucci C, Donga P, et al. Adherence and persistence
patterns in medication use among men with lower urinary tract symptoms/
benign prostatic hyperplasia. Am J Mens Health. 2017;11(1):164-169.
112. Ababneh M, Shamieh D, Al Demour S, et al. Evaluation of the
clinical pharmacist role in improving clinical outcomes in patients with
lower urinary tract symptoms due to benign prostatic hyperplasia. Int J
Clin Pharm. 2019;41:1373-1378.
113. Hotz T, Zhou MT, Reissmann ME, et al. Assessing the readability
and quality of online information about benign prostatic hyperplasia.
World J Urol. 2023;41(1):257-262.