Managing Opioid Use for Acute Pain in Pediatric Outpatient Settings
RELEASE DATE
March 1, 2025
EXPIRATION DATE
March 31, 2027
FACULTY
Olga Hilas, PharmD, MPH, EdS, BCPS, BCGP, FASCP
Professor, Department of Pharmacy Practice
St. John’s University
College of Pharmacy and Health Sciences
Queens, New York
Sarah S. Dezelic, PharmD, BCPPS
Associate Professor, Department of Pharmacy Practice
St. John’s University
College of Pharmacy and Health Sciences
Queens, New York
FACULTY DISCLOSURE STATEMENTS
Drs. Hilas and Dezelic have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-25-016-H08-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To update pharmacists on recent guidance from the American Academy of Pediatrics regarding the management of acute pain with opioids among children and adolescents in outpatient settings.
OBJECTIVES
After completing this activity, the participant should be able to:
- Describe the challenges associated with assessing and treating pain in children and adolescents.
- Explain the potential consequences of inadequate pain management in pediatric patients.
- Review existing guidance documents and reports on pediatric pain management.
- Discuss key recommendations from the 2024 American Academy of Pediatrics clinical practice guideline on opioid use for acute pain in children.
ABSTRACT: Assessment and treatment of pain among children and adolescents remains a challenge for healthcare practitioners, patients, and caregivers. Inadequate identification and management of pain in this age group can lead to serious consequences, such as delayed wound healing, immune dysfunction, decreased pain threshold, and chronic pain. Guidance documents and reports for the treatment of pain with analgesics (including opioids) have been published by several professional organizations; however, these guidelines and recommendations have focused on pediatric patients with specific disease states and/or within inpatient settings. In 2024, the first clinical practice guideline for the treatment of acute pain with opioids in children and adolescents in outpatient settings was released by the American Academy of Pediatrics, providing key recommendations for optimal patient care.
Pain experienced by infants, children, and adolescents accounts for over 50% of urgent visits to the pediatrician’s office.1 Pediatric patients often experience accidents and injuries from activities related to their developmental stage. Toddlers are at the greatest risk for falling, school-aged children are at the greatest risk for bicycle-related injuries, and adolescents experience more sports and recreation– or occupational-related injuries. A report from 2016 revealed that of 2.3 million children evaluated in emergency departments following a fall, only 2.9% were admitted for inpatient care.2 Most minor injuries are managed at home by caregivers and pediatricians—not in hospitals. While accidental injuries such as minor burns, sprains, and broken bones are the most obvious reason for pain, children and adolescents seek pain relief from headaches, infections, and dysmenorrhea. Minor surgical procedures and dental work are the most common sources of moderate-to-severe pain for the pediatric population needing management with opioids.3
Pain experienced by children and adolescents has been underrecognized and subsequently undertreated.4 Inadequate pain control delays wound healing, promotes immune dysfunction, and lowers the pain threshold for future experiences.5 One study of pediatric patients evaluated in emergency departments for pain compared patient self-assessment of pain with physician and parenteral assessments of pain.6 Both parents and physicians underestimated pain by children of all ages; however, pain experienced by children aged 8 years and younger was the most significantly underestimated by parents and physicians. In addition, of the 19 children who reported severe pain, only 14.3% received pain medication.6
Until recently, a common belief was that infants and children did not experience pain to the same degree as adults.7 This has resulted in knowledge gaps related to the pain experienced by infants, children, and adolescents, as well as inadequate assessments of pain in pediatric patients and appropriate treatment modalities in the outpatient setting. Research on the inpatient management of pain experienced by neonates, infants, children, and adolescents has demonstrated that pediatric patients experience pain similarly to adults.7 One exception is during the neonatal period, when the central nervous system pathways intended to inhibit painful input are immature and unable to dampen pain signals. Neonates actually experience an exaggerated response to painful stimuli.7 Even with physiological models illustrating pain mechanisms, the treatment of pain in pediatric patients is still unsatisfactory. Studies show that adults are more than twice as likely to receive opioid medication for the management of moderate-to-severe pain than children with moderate-to-severe pain.6-8 Also, undertreatment of acute pain experienced by youth results in 30% developing chronic pain, similar to the percentage seen in adults.8 Additional attention to the management of pain experienced by pediatric patients in the inpatient setting has resulted in improved assessment and multiple modalities to manage acute and chronic pain.
For example, there are recent publications detailing the role of lidocaine for the management of postoperative pain, chemotherapy-induced neuropathic pain, hyperalgesia, visceral pain, and centrally mediated pain in pediatric patients.9 Lidocaine has been shown to treat both acute and chronic pain when administered as a short-course continuous infusion. Optimal dosing, duration of therapy, and intervals of treatment have yet to be determined.9 The use of methadone for the prevention of iatrogenic withdrawal syndrome in critically ill pediatric patients has increased over the past decade. The long half-life, high enteral bioavailability, and additional N-methyl-D-aspartic acid–receptor antagonism of methadone prevent the emergence of troublesome withdrawal symptoms and the development of tolerance.10 Finally, sickle cell pain management guidelines recommend ketamine as a continuous infusion for pain that is not adequately treated by opioids.11
PAIN ASSESSMENT
Best practices for assessment of pain incorporate self-assessment and reporting of pain. Recognition of pain can be challenging in pediatric patients, especially when they are unable to effectively communicate. Children aged as young as 18 months begin to use pain words in their vocabulary.12 By age 4 years, children are able to verbalize their pain through a self-assessment tool.1,3,7,8 Currently, over 60 self-reported pain tools are available, of which three are recommended for use: Numeric Rating Scale–11 (NRS-11), Faces Pain Scale–Revised (FPS-R), and Color Analogue Scale (CAS).4 All three are validated tools for self-assessment of acute pain in the inpatient setting. NRS-11 can be verbally administered to children aged 6 years and older.1,3,8 FPS-R is valid for children aged 2 to 7 years and CAS for children aged 8 years and older. FPS-R also places less emphasis on emotion compared with the traditional Wong-Baker Faces Scale.1 Only six of the 55 available observational pain instruments are recommended for use in the inpatient setting (see TABLE 1).1,4,13 Two of these have been validated for procedural pain, three for assessing postoperative pain, and one (the Modified Behavioral Pain Scale) for assessing immunizations-related pain.4
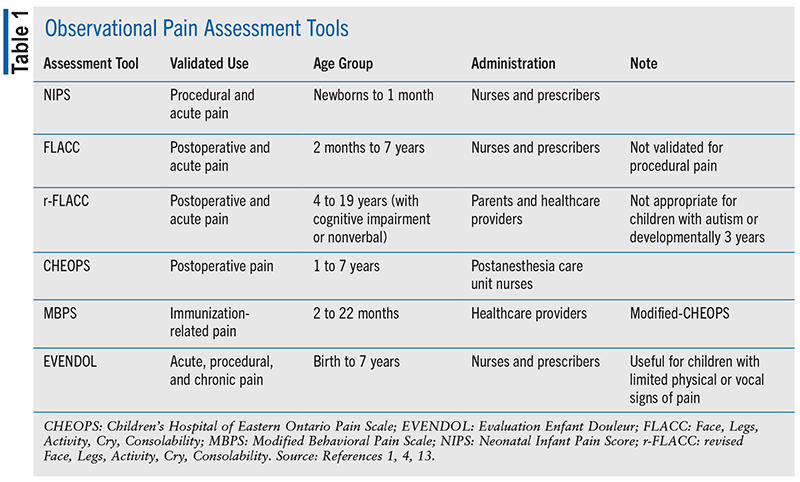
Observational assessment of pain in children unable to communicate includes the use of validated scales and distinguishing pain behaviors from those that are considered a natural part of the underlying condition.13 Tools appropriate for pain assessment of nonverbal children with neurologic impairment include the revised Face, Legs, Activity, Cry, Consolability (r-FLACC), Non-Communicating Children’s Pain Checklist–Revised (NCCPC-R), and the Pediatric Pain Profile (PPP). One tool is not recommended over the others.13 Pain assessment tools should be selected based on validation, ease of use, and developmental age of the patient.
MANAGEMENT APPROACHES
The management of pain in outpatient settings has not been explored as extensively as in inpatient settings. The barriers to identifying and optimally managing pain experienced by children in outpatient settings have been particularly complex due to the lack of clinical guidelines for the assessment and communication of pain management among this patient population.12 Pain is subjective and may be influenced by culture, language, and previous experiences.4 A recent study demonstrated that acculturated English-speaking Hispanic parents are more wary of nontraditional Hispanic and conventional modalities to treat pain.14 This medical-cultural confusion makes these parents more likely to undertreat their child’s pain when at home.4,14 Cultural expectations influence the reporting of experienced pain. In certain cultures, the comfort of others is considered before self-comfort; therefore, crying is discouraged, and pain behaviors are muted out of consideration for others.1
Prescribers should tailor medications for pain management to such considerations, as well as the resources and environment in which the patient is being treated. Caregivers administering the medication should be educated about how to use the medication, adverse effects, and when to seek help. Instructions must be clear, and understanding must be assessed by using the teach-back method to ensure optimal pain management. Otherwise, caregivers and patients may avoid taking prescribed medications due to fear of addiction or misconceptions about pain.15,16 Selecting a dosage form that is developmentally appropriate and available is imperative for the successful treatment of pain in a pediatric patient. Not all children can swallow tablets or capsules; some will refuse poor-tasting medications, requiring hospital admission for parenteral therapy. Patient and caregiver preferences and availability of support systems must be considered when determining the optimal regimen.12 Discussions addressing medication-related issues should be incorporated into treatment plans to prepare patients and caregivers for the management of acute pain at home. The plan should be reinforced at each point of contact with the healthcare system.
Guidance for prescribers on how to appropriately assess and address pain, personalize medication regimens, and educate all involved is needed.8,17 In 2017, the FDA issued a safety communication restricting the use of codeine and tramadol in children aged 12 years and younger.18 However, in 2019, codeine was dispensed over 45,000 times, and tramadol was dispensed nearly 42,000 times to children aged 11 years or younger.3 In 2023, the FDA changed the warning on codeine and tramadol to a contraindication for use in children aged younger than 12 years. The FDA also issued new warnings against the use of codeine and tramadol in 1) children and adolescents aged <18 years for the treatment of pain after surgery to remove tonsils and/or adenoids, and 2) adolescents aged between 12 and 18 years who are obese and/or have serious respiratory conditions (e.g., obstructive sleep apnea or severe lung disease).18 The limitations placed on the prescribing of codeine and tramadol created another practice gap in the management of pain in the outpatient setting.18
SPECIAL CONSIDERATIONS
A survey was conducted among the parents of 185 pediatric patients who underwent ambulatory surgery to assess the usage of opioid analgesia and the management of leftover opioids.19 The median number of opioid doses prescribed was 12, while the median number of opioids administered was two. Furthermore, only 42% of parents who reported having unused opioids had disposed of them, while about one-third of those with opioids remaining stored them in a locked location. Unused opioid medications and active opioid prescriptions of other family members are the most common sources of opioid access in adolescents and young adults.20 Up to 15% of families report living with a family member who has a substance use disorder.21,22 Fortunately, nonmedical use of opioids in adolescents ages 12 to 17 years has decreased from 3.9% in 2015 to 2.3% in 2019.20 Harm reduction methods, such as storing medications in a locked container, decrease diversion rates.20,23 Providing safe disposal options, such as drug disposal bags or reminders to dispose of leftovers, significantly improves opioid disposal rates.23
Following acute illness or injury, patients and their caregivers receive many instructions for managing the injury, surgical site, pain, and follow-up appointments. For example, less than 50% of youth initially seen in the emergency department for mild traumatic brain injury seek the recommended follow-up care, and 20% of their caregivers were unable to recall three postconcussive symptoms after discharge planning.21 Cognitive overload impairs recall of medication directions, even with visual cues.24,25 Reinforcement of how to properly use opioid medications by pharmacists improves understanding of medication administration and adverse effects management.25
PREVIOUS GUIDELINES
Pediatric subspecialists have developed pain management guidelines over the past 5 years with the goals of harm reduction and optimal postoperative pain management. In 2020, evidence-based guidelines for prescribing opioids in pediatric patients after surgery were published.19 These guidelines underscore the importance of limiting opioid duration of therapy to minimize opioid misuse and dependence in pediatric and adolescent patients. The guidelines are often divided into into two categories: opioid-free recovery is recommended, and those when opioid-free recovery is possible. In 2020, the American Society of Hematology developed evidence-based guidelines for the management of acute and chronic pain experienced by children and adults with sickle cell disease.11 Subsequently, the Society of Critical Care Medicine published clinical practice guidelines for the management of pain, agitation, neuromuscular blockade, and delirium in critically ill pediatric patients, and the American Dental Association (ADA) created evidence-based guidelines for the management of acute dental pain in children.26,27 The ADA specifically recommends the use of nonsteroidal anti-inflammatory drugs alone or in combination with acetaminophen for toothaches in children following proper assessment of the pain using a suitable tool for children, in addition to engaging in discussions with caregivers regarding preferences, past experiences, and values.
AAP CLINICAL PRACTICE GUIDELINE
In November 2024, the American Academy of Pediatrics (AAP) published the first clinical practice guideline to support the appropriate use of opioids for acute pain in pediatric patients in the outpatient setting.17,28 This guideline was based on the comprehensive appraisal of randomized clinical trials (RCTs) and systematic reviews (SRs) involving outpatient prescribing of opioids among pediatric patients conducted between 2010 and 2023 and identified using the PubMed and Embase databases.
Methodology
A search protocol was developed to include all RCTs and SRs from January 2010 to June 2023 identified through PubMed and Embase. Inclusion criteria required literature retrieved to include children aged <18 years with acute pain prescribed an opioid for home use (in certain cases up to age 21 years if stratified from older adults). Studies excluded the lack of outcomes in home settings and/or assessed 1) opioids administered only in emergency departments, urgent care centers, or inpatient centers; 2) opioid treatment for neonatal opioid withdrawal syndrome; and 3) opioid use for acute episodes of pain associated with chronic medical conditions such as sickle cell disease, cancer, or palliative care.28
The opioid guideline panel (which consisted of pediatric primary and tertiary care providers, pediatric experts in pain medicine, emergency medicine, toxicology, surgery, adolescent and addiction medicine, study methodologists, implementation scientist, and parent representative) engaged health librarians and designed the literature review to focus on 1) opioid prescribing in children experiencing acute pain in the outpatient setting compared with other pharmacologic and nonpharmacologic treatments or between opioids and 2) individual-, family-, or health systems–level interventions that aim to increase safe outpatient/ambulatory opioid prescribing for children compared with standard practice. Outcomes assessed included short-term safety, short-term efficacy and effectiveness, persistent postoperative or postprocedural pain (>2 weeks later), unintentional ingestion, diversion, misuse, excess opioid medication, opioid-related overdose, addiction, and incident or recurrent opioid use disorder (within 1 year of prescription).28
Recommendations
The AAP Clinical Practice Guideline on opioid prescribing for acute pain management in children and adolescents in outpatient settings emphasizes the importance of a multimodal approach to acute pain management that includes use of appropriate nonpharmacologic therapies, nonopioid medications, and opioid medications (when needed for acutely worsening pain or in patients with a history of chronic pain).17 It cautions against the use of opioids as monotherapy or in combination with other sedating medications in children or adolescents with acute pain in outpatient settings. If opioids are prescribed for treatment among patients in these age groups, they should be started with immediate-release formulations at the lowest age- and weight-appropriate doses for initial durations of <5 days. In cases where pain management is warranted for surgery or trauma, longer durations may be appropriate. Discontinuation or rapid tapering of opioids may be harmful, particularly among patients who have used these medications long-term for chronic pain management.
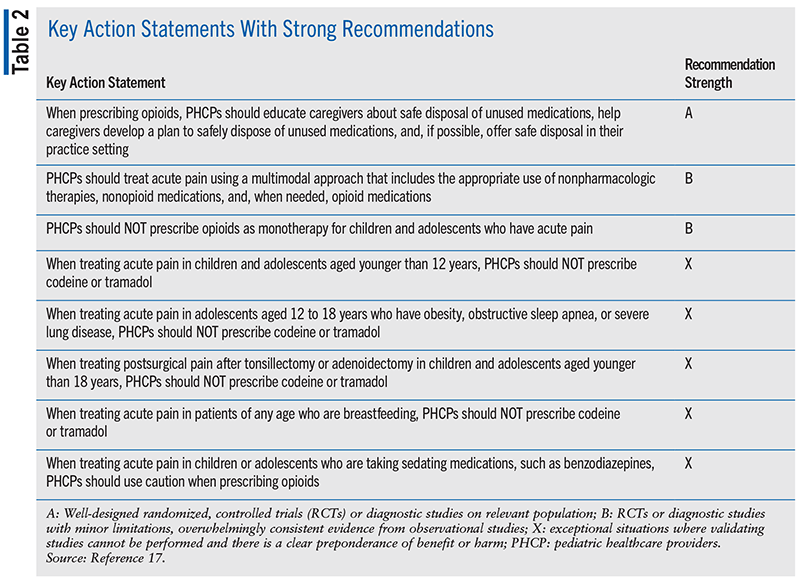
The guideline also states that codeine and tramadol should not be prescribed for patients who are 1) aged <12 years; 2) aged 12 to 18 years with obesity, obstructive sleep apnea, or severe lung disease; 3) aged <18 years to treat pain following tonsillectomy or adenoidectomy; or 4) breastfeeding. Patients, caregivers, opioid-prescribing clinicians, and/or specialists in pain management should all be involved in the development of appropriate treatment plans. These plans should include the provision of counseling on the proper use, safe storage, and disposal of opioid medications and direct observation of opioid administration (if needed) to patients and caregivers. In addition, the opioid antagonist naloxone and education on naloxone should be provided to all patients receiving an opioid for the treatment of pain. TABLE 2 provides a summary of the key action statements with strong recommendations within the 2024 AAP Clinical Practice Guideline.
CONCLUSION
The treatment of acute pain among children and adolescents in outpatient settings requires a thoughtful and efficient approach. Many minor injuries can be adequately managed with OTC analgesic medications. The traditional tenets of pain management (in accordance with the World Health Organization analgesic ladder), scheduling doses when pain is expected to be ongoing and leveraging multiple modalities to provide analgesic relief, all apply to the pediatric population.7 Pharmacists must assess the safety of each patient’s prescribed analgesic medication by verifying the dose per unit of weight, frequency, duration, and dosage form. In the outpatient setting, fewer healthcare providers are involved in reviewing and managing the patient’s pain regimen compared with the inpatient setting. Therefore, ensuring the safe use of opioid prescriptions in outpatient settings is paramount. Interventions related to drug interactions, adjusting doses of opioids in obese patients, and ensuring that the caregiver understands how to use the opioid medication, as well as naloxone coprescribing, support the safe and effective use of opioids for outpatient pain management.17,19,29,30
REFERENCES
- Villacres S, Chumpitazi CE. Acute pediatric pain management in the primary care office. Pediatr Ann. 2018;47(3):e124-e129.
- Gettel CJ, Schuur JD, Mullen JB, Venkatesh AK. Rising high-acuity emergency care services independently billed by advanced practice providers, 2013 to 2019. Acad Emerg Med. 2023;30(2):89-98.
- Chua KP, Brummett CM, Conti RM, Bohnert AS. Opioid prescribing to U.S. children and young adults in 2019. Pediatrics. 2021;148(3):e2021051539.
- Trottier ED, Ali S, Doré-Bergeron MJ, Chauvin-Kimoff L. Best practices in pain assessment and management for children. Paediatr Child Health. 2022;27(7):429-448.
- Groenewald CB. Opioid-prescribing patterns for pediatric patients in the United States. Clin J Pain. 2019;35(6):515-520.
- Brudvik C, Moutte SD, Baste V, Morken T. A comparison of pain assessment by physicians, parents and children in an outpatient setting. Emerg Med J. 2017;34(3):138-144.
- Tobias JD. Acute pain management in infants and children–part 1: pain pathways, pain assessment, and outpatient pain management. Pediatr Ann. 2014;43(7):e163-e168.
- Holley AL, Gaultney W, Turner H, Wilson AC. The Pediatric Pain Screening Tool (PPST) can rapidly identify elevated pain and psychosocial symptomatology in treatment-seeking youth with acute musculoskeletal pain. J Pain. 2022;23(1):65-73.
- Hall EA, Sauer HE, Davis MS, Anghelescu DL. Lidocaine infusions for pain management in pediatrics. Paediatr Drugs. 2021;23(4):349-359.
- Smith HAB, Besunder JB, Betters KA, et al. 2022 Society of Critical Care Medicine clinical practice guidelines on prevention and management of pain, agitation, neuromuscular blockade, and delirium in critically ill pediatric patients with consideration of the ICU environment and early mobility. Pediatr Crit Care Med. 2022;23(2):e74-e110.
- Brandow AM, Carroll CP, Creary S, et al. American Society of Hematology 2020 guidelines for sickle cell disease: management of acute and chronic pain. Blood Adv. 2020;4(12):2656-2701.
- Pope N, Tallon M, McConigley R, et al. Experiences of acute pain in children who present to a healthcare facility for treatment: a systematic review of qualitative evidence. JBI Database System Rev Implement Rep. 2017;15(6):1612-1644.
- Hauer J, Houtrow AJ; AAP Section on Hospice and Palliative Medicine, Council on Children with Disabilities. Pain assessment and treatment in children with significant impairment of the central nervous system. Pediatrics. 2017;139(6):e20171002.
- Fortier MA, Martin SR, Kain DI, Tan ET. Parental attitudes regarding analgesic use for children: differences in ethnicity and language. J Pediatr Surg. 2011;46(11):2140-2145.
- Williams G. What dilemmas do healthcare workers face looking after children with acute pain? Pain Manag. 2017;7(4):279-286.
- Ali S, Dworsky-Fried Z, Moir M, et al. Factors influencing parental decision-making regarding analgesia for children with musculoskeletal injury-related pain: a qualitative study. J Pediatr. 2023;258:113405.
- Hadland SE, Agarwal R, Raman SR, et al. Opioid Prescribing for Acute Pain Management in Children and Adolescents in Outpatient Settings: clinical practice guideline. Pediatrics. 2024;154(5):e2024068752.
- FDA. FDA drug safety communication: FDA restricts use of prescription codeine pain and cough medicines and tramadol pain medicines in children; recommends against use in breastfeeding women. October 19, 2023. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-restricts-use-prescription-codeine-pain-and-cough-medicines-and. Accessed January 7, 2025.
- Kelley-Quon LI, Kirkpatrick MG, Ricca RL, et al. Guidelines for opioid prescribing in children and adolescents after surgery: an expert panel opinion. JAMA Surg. 2021;156(1):76-90.
- Chadi N, Walker-Harding L; American Academy of Pediatrics Committee on Substance Use and Prevention. Nonmedical use of controlled medications by adolescents and young adults: clinical report. Pediatrics. 2024;154(6):e2024069298.
- Pham TN, Morrison AK, Menard MS, et al. Using comic-based concussion discharge instructions to address caregiver health literacy in the emergency department. J Emerg Nurs. 2023;49(2):236-243.
- Sebastian S, Thornton H, Abdel-Rasoul M, Shaffery K. Patient-perceived understanding of home-going medication with transitions of care services at a pediatric institution. J Am Pharm Assoc. 2024;64(3):102076.
- Stone AL, Qu’d D, Luckett T, et al. Leftover opioid analgesics and disposal following ambulatory pediatric surgeries in the context of a restrictive opioid-prescribing policy. Anesth Analg. 2022;134(1):133-140.
- Latorre-Postigo JM, Ros-Segura L, Navarro-Bravo B, et al. Older adults’ memory for medical information, effect of number and mode of presentation: an experimental study. Patient Educ Couns. 2017;100(1):160-166.
- Thigpen JC, Odle BL, Harirforoosh S. Opioids: a review of pharmacokinetics and pharmacodynamics in neonates, infants, and children. Eur J Drug Metab Pharmacokinet. 2019;44(5):591-609.
- American Dental Association. New guideline details dental pain management strategies for pediatric patients. August 25, 2023. www.ada.org/about/press-releases/new-guideline-details-dental-pain-management-strategies-for-pediatric-patients. Accessed January 7, 2025.
- Carrasco-Labra A, Polk DE, Urquhart O, et al. Evidence-based clinical practice guideline for the pharmacologic management of acute dental pain in children: a report from the American Dental Association Science and Research Institute, the University of Pittsburgh School of Dental Medicine, and the Center for Integrative Global Oral Health at the University of Pennsylvania. J Am Dent Assoc. 2023;154(9):814-825.e2.
- Raman SR, Smith MJ. Evidence for the use of opioid medication for pediatric acute pain in the outpatient setting: technical report. Pediatrics. 2024;154(5):e2024068753.
- Trottier ED, Doré-Bergeron MJ, Chauvin-Kimoff L, et al. Managing pain and distress in children undergoing brief diagnostic and therapeutic procedures. Paediatr Child Health. 2019;24(8):509-535.
- American Academy of Pediatrics. AAP releases clinical practice guideline for opioid prescriptions. September 30, 2024. www.healthychildren.org/English/news/Pages/american-academy-of-pediatrics-releases-clinical-practice-guideline-for-opioid-prescriptions.aspx. Accessed December 10, 2024.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.