Newer Agent Use in Obese and Overweight Adults
RELEASE DATE
December 1, 2024
EXPIRATION DATE
December 31, 2026
FACULTY
Katherine Hale, PharmD, BCPS, MFA
Freelance Medical Writer
Tri-Cities, Washington
Sherrill Brown, DVM, PharmD, BCPS
Professor, Pharmacy Practice
Skaggs School of Pharmacy
University of Montana
Missoula, Montana
FACULTY DISCLOSURE STATEMENTS
Dr. Hale and Dr. Brown have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-24-121-H01-P
Credits: 2.0 hours (0.20 ceu)
Type of Activity: Knowledge
TARGET AUDIENCE
This accredited activity is targeted to pharmacists. Estimated time to complete this activity is 120 minutes.
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients' conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
GOAL
To update pharmacists regarding current concepts in adult weight management, with a focus on newer agents.
OBJECTIVES
After completing this activity, the participant should be able to:
- Discuss the risk factors, assessment, and diagno- sis of overweight and obesity.
- List nonpharmacologic and surgical interven- tions for weight management.
- Identify appropriate pharmacologic interven- tions for a patient with obesity.
- Describe the role of the pharmacist in weight management.
ABSTRACT: With more than 40% of U.S. adults affected by obesity and an additional 31% considered overweight, the weight-management landscape is constantly evolving. In addition to metabolic, physical, and psychosocial complications, obesity and overweight result in increased healthcare expenditures. Lifelong individualized management is necessary due to the multifactorial causes of obesity and overweight. In conjunction with lifestyle modifications, pharmacologic and surgical methods may be used to achieve weight-loss goals. Newer agents, such as glucagon-like peptide-1 (GLP-1) receptor agonists and combination GLP-1 receptor/glucose-dependent insulinotropic polypeptide agonists, provide significant weight-loss benefits, but patients have been challenged by accessibility issues due to cost/coverage and drug shortages.
Obesity affects more than 40% of the U.S. adult population, and 9% have severe obesity. An additional 31% of U.S. adults are overweight.1 Medical costs are higher for people with obesity. In 2019, obesity was responsible for $173 billion in medical expenditures. Adults with obesity spend almost $2,000 more annually on healthcare than healthy-weight adults; for those with severe obesity, healthcare costs increase by $3,000.2 Obesity, the accumulation of excessive fat, is a chronic disease that results in metabolic, physical, and psychosocial complications.3,4 Patients with obesity have an increased risk of many diseases, including type 2 diabetes (T2DM), obstructive sleep apnea, nonalcoholic fatty liver disease, cardiovascular disease (CVD), osteoarthritis, and colon, esophageal, renal, and other cancers. 4-7
The etiology of obesity is multifactorial—biological factors including genetics, neurohormonal mechanisms, other chronic diseases, sociocultural practices and beliefs, social determinants of health, childhood experiences, psychological factors such as binge-eating disorders, feelings of self-worth and identity, and mood and anxiety disorders.6 In addition, many medications can promote weight gain, for example, atypical antipsychotics such as olanzapine. As a result, the treatment of obesity should be individualized and will require lifelong support.6
Obesity is often staged using BMI, an estimate of total body fat mass. BMI is calculated by dividing body weight in kilograms by height in meters squared.5 The stages of obesity are provided in TABLE 1. It is important to be aware of the limitations of BMI when used as a screening tool for weight loss.4,8 BMI does not distinguish between fat and lean body mass, nor does it distinguish between subcutaneous and visceral fat. BMI is not accurate when used for older adults, those with high muscle mass, or adults of Asian descent. 4,8
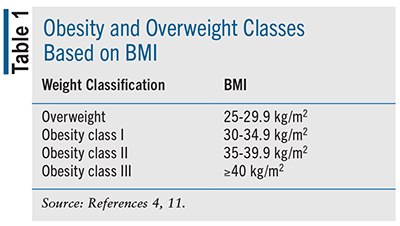
Since BMI is not a perfect measure of body fat mass, other assessments are available for diagnosis and monitoring of obesity, such as waist circumference, waist-to-hip ratio, and waist-to-height ratio.4 Waist circumference assesses the amount of abdominal fat, which is strongly associated with CVD risk, all-cause mortality, and cardiovascular (CV) mortality.8 Waist circumference ≥40 inches (102 cm) in men and ≥35 inches (88 cm) in women is associated with an increase in disease risk.5,8,9
While the usual goal of weight loss in patients with obesity is 5% of the baseline body weight, any magnitude of weight loss is beneficial.4 In patients with diabetes, loss of 3% to 7% of baseline weight is associated with improvements in glycemia, blood pressure, and lipid levels. The need for antihyperglycemic medications may decrease as a result. With a sustained weight loss of >10%, patients may experience remission of T2DM and improvement of long-term CV outcomes.4 For every kilogram of weight lost, the risk of developing diabetes decreased by 16% in the Diabetes Prevention Program study.10 Weight loss of 5% to 10% or more leads to improvements in urinary incontinence, sleep apnea, and gastroesophageal reflux symptoms and reductions in A1C, triglycerides, blood pressure, and medications for diabetes and hypertension. In women with polycystic ovary syndrome, ovulation and regular menses are likely with 10% or greater weight loss, improving fertility.5,11
There are three approaches to the treatment of obesity: lifestyle changes (diet and exercise), pharmacologic therapy (prescription and nonprescription medications), and bariatric surgery and devices.4,7,9,12,13 Regardless of approach, treatment goals are similar: improvement of comorbid conditions and obesity-related diseases, improvement in the psychological well-being of the patient, and potential cosmetic benefits.14
Guidelines on treatment of obesity in adults differ slightly in their recommendations, but most recommend the development of an individualized approach consisting of diet modification or restrictions, increased physical activity, and behavior therapy.4-7,9,12 Bariatric surgery can be recommended to patients with class III obesity (BMI ≥40 kg/m 2 ). Patients with lower BMI who have other comorbidities may also be candidates for surgery, specifically patients with T2DM and class I obesity and those with class II obesity in addition to an obesity-related comorbidity.5-7 Pharmacologic therapy is usually an option, and some guidelines, such as those from the European Society of Cardiology in 2021, endorse specific agents.4-7,9,12,13 However, no obesity guidelines have been updated recently enough to evaluate the effectiveness and safety of the newer agents, semaglutide (Wegovy) and tirzepatide (Zepbound).
NONPHARMACOLOGIC MANAGEMENT OF OBESITY
Lifestyle Interventions
As mentioned, lifestyle interventions are a mainstay of weight-loss management.4-7,9,12 Diet modification or restriction is the most common weight-loss strategy, and the goal is at least a 500 kcal/day energy deficit. A net caloric deficit of 500 to 1,000 kcal/day can result in weight loss of 0.5 to 2 pounds per week, which is a realistic initial weight-loss goal.7 Plant-based, Mediterranean, low-carbohydrate, very low-carbohydrate, and low-fat diets are commonly recommended for short-term weight loss. The Mediterranean diet often has long-term benefits.7,9 Low-carbohydrate diets (≤40% of calories from carbohydrates or ≤120 g carbohydrates/day) may be preferable to low-fat diets (<30% of energy from fat) for short-term weight loss (up to 6 months) and to lower cholesterol.7 While low- or very-low-car- bohydrate diets help with appetite control, they may be ketogenic; therefore, medical or dietetic supervision is recommended.4,9 Very-low-carbohydrate diets should not be continued long-term.9
Nutritional supplements, such as botanical products, high-dose vitamins, amino acids, enzymes, and antioxidants, are not effective for weight loss.4 However, vitamin and mineral supplementation should be recommended to treat deficiencies. Protein supplements may be used in conjunction with medically supervised weight-loss therapies.4 Commercial programs (e.g., Jenny Craig, Nutrisystem) offer structured diets and support systems to assist with weight loss. These programs may improve A1C compared with dietary counseling alone but have not been shown to improve blood pressure or lipids.6
Physical activity can help patients achieve small amounts of weight and fat loss and can result in decreased abdominal fat even without weight loss.4-7,9,12 In addition, exercise is good for maintaining muscle mass during weight loss and for maintaining weight loss. Aerobic exercise improves CV fitness, and resistance training promotes weight maintenance, increases muscle mass, and improves motility.6,9 The recommended amount of aerobic activity is ≥150 minutes per week of moderate exercise divided into three to five daily sessions per week. Resistance training is recommended two to three times per week.9
Behavior therapy includes self-monitoring of weight, food intake, and exercise.4,5,7,9 In addition, dietary education, assistance with goal setting, stress reduction techniques, cognitive restructuring, and individual or group meetings can be used as part of a comprehensive lifestyle intervention.4,5,7,9 Behavior interventions should be intensified for patients who do not lose 2.5% of their baseline weight in the first month since early weight loss is indicative of long-term success.9
Bariatric Surgery and Devices
Bariatric surgery can lead to significant weight loss and improve obesity-related conditions such as T2DM.4 Both the vertical sleeve gastrectomy and the Roux-en-Y gastric bypass rapidly improve hyperglycemia and glucose homeostasis in patients with diabetes.4 Due to insufficient evidence in patients aged older than 65 years, bariatric surgery should not be routinely recommended in this population. 7
While potential weight loss with bariatric surgery may be attractive to patients, surgical complications can occur.4 Major complications occur in 2% to 6% of patients, and up to 15% will experience minor complications or the need for additional surgery. The long-term consequences of bariatric surgery include vitamin and mineral deficiencies requiring lifelong supplementation, anemia, and osteoporosis. Dumping syndrome may occur immediately after surgery. Patients may experience diarrhea, nausea, vomiting, palpitations, and fatigue approximately 10 to 30 minutes after a meal. Severe hypoglycemia may occur more than a year after surgery because of altered gastric emptying and rapid glucose absorption in the gastrointestinal (GI) tract, leading to excessive excretion of glucagon-like peptide-1 (GLP-1) and overstimulation of insulin release. Patients experience hypoglycemia 1 to 3 hours after ingesting a high-carbohydrate meal.4
The FDA has approved gastric balloons and vagus nerve stimulators for short-term weight loss.4 The high cost and limited efficacy data, especially in patients with T2DM, limit the use of these devices.4 However, gastric balloons used for less than 6 months have resulted in greater weight loss and lower BMI and waist circumference than lifestyle interventions alone.7 Patients may have mild adverse effects (nausea, vomiting, abdominal pain, gastroesophageal reflux), but they may achieve control of T2DM. Gastric aspiration therapy with percutaneous gastrostomy devices produces modest short-term weight loss. This therapy requires the removal of gastric contents after a meal. While the adverse effects are minimal (pain, gastric reflux), this technique may promote continued poor lifestyle habits.7 Gastric banding devices are not recommended for weight loss due to their limited long-term efficacy and high rate of complications.4
NONPRESCRIPTION PRODUCTS
FOR WEIGHT LOSS
Many natural medicines are marketed for weight loss.15 Unfortunately, data supporting the efficacy and safety of natural products can be scarce and are often subject to bias. A recent systematic review and meta-analysis included 54 randomized, controlled trials of herbal supplements for weight loss. Only Camellia sinensis (green tea) in combination with other agents and Phaseolus vulgaris (white kidney bean), both in combination and as a single ingredient, were associated with statistically significant weight loss compared with placebo in the meta-analysis. With both herbs, the weight loss was <5 kg and, therefore, was not clinically significant. Other natural medicines had positive results in a small number of studies (≤3 studies), but most of the studies were limited by poor reporting of the intervention, the study design and methodology, and the lack of safety data. Many studies were less than 4 months in duration, so long-term effects of the products remain unknown. Natural products often contain multiple ingredients, so it is difficult to ascertain if a single ingredient is effective for weight loss.15 Ephedra (Ephedra sinica) effectively promoted weight loss in five studies, but the potential for severe CV effects led to the banning of ephedra and ephedrine alkaloids by the FDA in 2004.15,16
OTC preparations of laxatives and diuretics may be used for weight loss.14 Diuretics may produce initial weight loss, but the effects are usually transient. Laxatives do not produce weight loss since the main effect is in the colon and not in the small intestine—the site of food absorption. Chronic use of laxatives (>2 weeks) may cause cathartic colon (inability to have regular bowel movements without the laxative) and electrolyte imbalances14.
FDA-APPROVED DRUGS FOR WEIGHT LOS
The FDA has approved several medications for weight loss—benzphetamine (Didrex), diethylpropion (Tenuate), orlistat (Xenical, alli), phendimetrazine (Bontril), phentermine (Ionamin), phentermine with topiramate (Qsymia), naltrexone with bupropion (Contrave), liraglutide (Saxenda), semaglutide (Wegovy), and tirzepatide (Zebound).17 Setmelanotide (Imcivree) is indicated only in patients with obesity due to either Bardet-Biedl syndrome or an abnormality of monogenic obesity genes (POMC, PCSK1, LEPR).18 Two weight-loss medications—sibutramine (Meridia) and lorcaserin (Belviq)—have been withdrawn from the market in the U.S. due to safety concerns.19,20
FDA-approved prescription medications for weight loss are indicated in patients with a BMI ≥30 kg/m 2 and in patients with a BMI ≥27 kg/m2 plus an obesity-related comorbid condition (e.g., diabetes, hypertension, dyslipidemia).17 Nonprescription orlistat is indicated for overweight adults (BMI ≥25 kg/ m 2 ). Weight-loss drugs should not be taken during pregnancy due to the potential harm to the fetus from maternal weight loss. These drugs should be used with caution during breastfeeding. All approved drugs for weight loss should be used in conjunction with lifestyle modifications (e.g., reduced-calorie diet and increased physical activity).17
Orlistat is a gastric and pancreatic lipase inhibitor, meaning that it decreases absorption of dietary fats.4,13,17 Orlistat 120 mg inhibits 30% of dietary fats and should be taken with meals containing ≥15 g of fat.17 When used with a reduced-calorie diet, orlistat 120 mg treatment for 6 months produced 4% more weight loss than placebo.4 With the 60-mg dose, most patients lose 5 to 10 pounds after 1 year.14 Orlistat lowers LDL cholesterol and blood pressure and promotes positive changes in eating habits, as patients alter their fat intake to decrease the adverse effects of the drug. Patients on orlistat are likely to have better adherence to diet modifications after discontinuing the medication than patients using appetite-suppressing drugs.14
Common adverse effects of orlistat are GI-related, such as flatulence, loose or frequent stools, fatty/oily stools, oily evacuation, and fecal urgency or incontinence.14,17 These effects usually resolve in the first few weeks of treatment and can be minimized by lowering the fat content of meals. Absorption of fat-soluble vitamins (A, D, E, and K) is decreased, and supplementation is required (separated from orlistat administration by 2 hours). Rare cases of severe liver injury have been reported with orlistat use, even with the lower nonprescription dose. Because orlistat has limited systemic absorption, drug interactions are not pharmacokinetic or pharmacodynamic but are due to impaired drug absorption. Orlistat is contraindicated in those with malabsorption disorders and should be used with caution in patients with thyroid disease, cholelithiasis, nephrolithiasis, or pancreatitis.14,17
Diethylpropion, benzphetamine, and phendimetrazine are sympathomimetic anorexiants approved for short-term (≤12 weeks) treatment for weight loss.13,17 These agents are not preferred for weight loss because of CV side effects (tachycardia, hyper-tension) and the potential for abuse.17 Other, preferred agents should be used instead. If used, patients should be evaluated after 4 weeks of treatment. If a clinically appropriate amount of weight has not been lost at that time, then diethylpropion and benzphetamine should be discontinued. Tolerance to appetite suppression has been reported with phendimetrazine, and the drug should be stopped if tolerance occurs.17
Phentermine is also a sympathomimetic anorexiant used for weight loss.4 It should be used for ≤12 weeks, although some researchers suggest longer durations in patients who experience weight loss without safety issues.13 In clinical studies lasting up to 24 weeks, patients on phentermine had a mean weight loss of 3.6 kg when compared with lifestyle interventions alone.13 Common side effects of phentermine are hypertension, tachycardia, restlessness, headache, insomnia, and dry mouth. Phentermine should not be used in patients with CVD, glaucoma, or hyperthyroidism or in those with a history of drug abuse.13,17
Phentermine combined with topiramate, a gamma-aminobutyric acid receptor modulator, in an extended-release capsule produces a synergistic reduction in appetite.13,17 The combination is indicated for chronic weight management and produced a mean 6.6-kg weight loss at the recommended 7.5-mg/46-mg dosage. With the higher 15-mg phentermine/92-mg topiramate dosage, the mean weight loss was 8.6 kg more than with lifestyle interventions alone.13 Side effects include insomnia, dry mouth, constipation, paresthesia, dizziness, and dysgeusia. Like phentermine, the combination is contraindicated in patients with hyperthyroidism and glaucoma. Patients should be monitored for depression or suicidal thoughts and serious skin conditions. If a rash occurs, the drug should be discontinued.13,17
The combination of an opioid antagonist and a dopamine/norepinephrine reuptake inhibitor (naltrexone/bupropion) appears to affect the appetite regulatory center and the reward system of the brain.13,17 Patients tend to lose 4% to 5% of their body weight within a year with naltrexone/bupropion. Adverse effects include nausea, constipation or diarrhea, headache, vomiting, dizziness, insomnia, and dry mouth.13,17 Suicidal thoughts and behavior may occur.17 Patients on opioid agonists, those with a history of seizures, uncontrolled hyper-tension, bulimia, or anorexia nervosa, and those undergoing acute opioid or alcohol withdrawal should not use naltrexone/bupropion.5,13,17 This medication should not be used in conjunction with linezolid or IV methylene blue. The chronic use of naltrexone may lower opioid dose requirements, so patients should be warned of the potential for opioid overdose; naltrexone/bupropion should be discontinued during opioid therapy.17
GLP-1 RAs
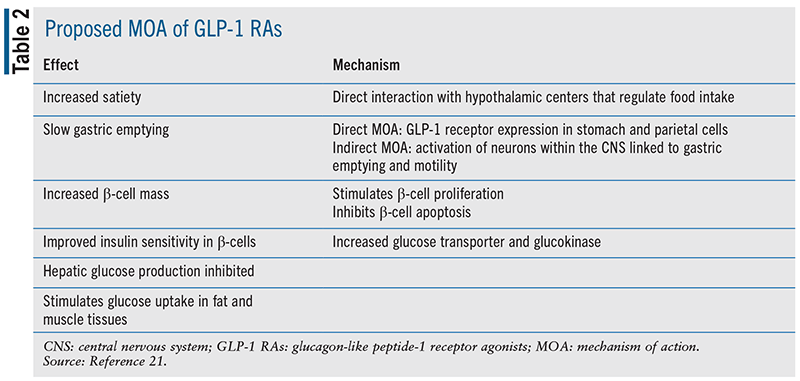
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are considered "incretin mimetics" because they mimic the action of GLP-1, an incretin hormone. Upon ingestion of high-fat foods and carbohydrates, GLP-1 is released from L-cells of the ileum and colon.21 GLP-1 receptors are found in a wide variety of tissues, such as the pancreas, stomach, intestine, heart, lung, kidney, pituitary, hypothalamus, brain stem, and the nodose ganglion neurons of the vagus nerve. The variety of receptor locations leads to multiple GLP-1 actions, such as slowed gastric emptying, increased satiety, decreased energy intake, increased β-cell proliferation, glucagon secretion inhibition, and increased insulin secretion (see TABLE 2). β-cell capacity to sense or respond to glucose is also improved. Upregulation of glucose transporters and glucokinases may cause previously insulin-resistant β-cells to become more sensitive.21
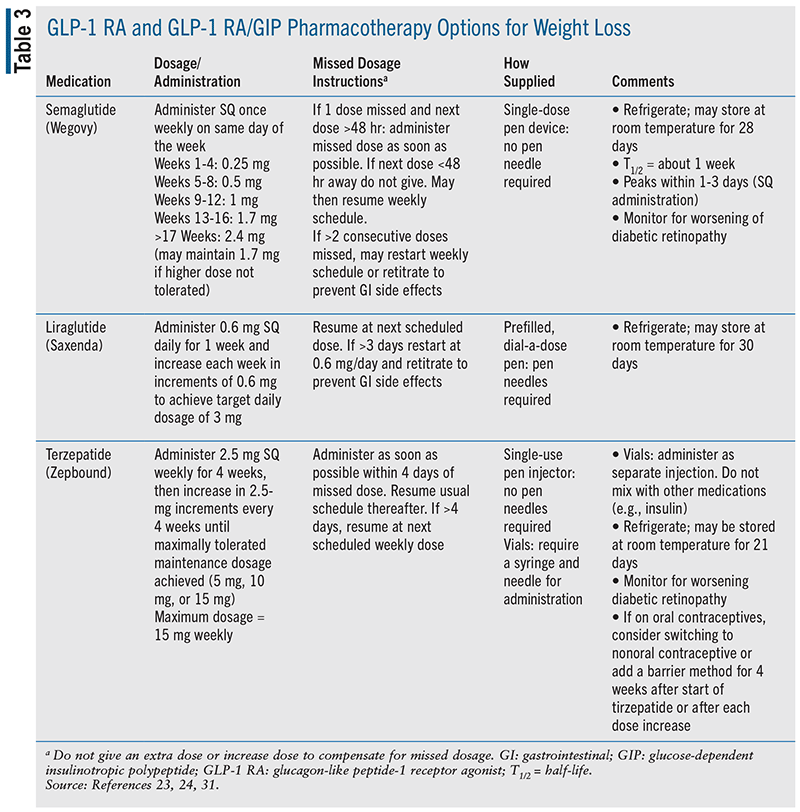
Prior to approval of injectable semaglutide (Wegovy) and injectable liraglutide (Saxenda) for weight loss, alternative dose formulations of injectable and oral semaglutide (Ozempic and Rybelsus) and injectable liraglutide (Victoza) were approved to treat elevated blood glucose in T2DM.17 A generic formulation is available for liraglutide but is FDA approved only for T2DM.22 The maximum doses of liraglutide and semaglutide used for weight loss are higher than those used for glycemic control in T2DM17 TABLE 3 summarizes GLP-1 RA options for weight loss.
Liraglutide
Administered as a daily SC injection, liraglutide (Saxenda) was approved in 2010 and is indicated in adults with BMI ≥30 kg/m 2 or BMI ≥27 kg/m 2 with at least one weight-related comorbid health condition. Liraglutide (Saxenda) may also be used in patients aged ≥12 years with body weight ≥60 kg and initial BMI corresponding to 30 kg/m 2 for adults using international cutoffs.23
Liraglutide titrated to a maximum dosage of 3 mg daily was evaluated in a randomized, double-blind, placebo-controlled trial in adults (N = 3,731) aged ≥18 years without T2DM with a BMI ≥30 kg/m 2 or a BMI ≥27 kg/m 2 and one weight-related condition (e.g., hypertension or dyslipidemia).25 Liraglutide 3 mg daily (N = 2,487) produced significantly more weight reduction (mean difference -5.6 kg; 95% CI -6.0 to -5.1; P <.001) compared with placebo (N = 1,244). The percertage of participants achieving 5% or 10% baseline body weight reduction with liraglutide (63.2% and 33.1%, respectively) was also significantly higher than with placebo (27.1% and 10.6%, respectively; P <.001).25
A study of adults diagnosed with prediabetes and BMI ≥30 kg/m 2 or BMI ≥27 kg/m 2 and one or more weight-related comorbidities found that greater weight loss was achieved with liraglutide at 3 years compared with placebo (-6.1 ± 7.3% vs. -1.9 ± 6.3%; 95% CI, -4.9 to -3.7; P <.0001). Weight-loss effects remained significant 12 weeks after treatment discontinuation (treatment difference -3.2%; 95% CI, -4.3 to -2.2; P <.0001).26
Semaglutide
Administered as a weekly SQ injection, semaglutide (Wegovy) was approved in 2017 and is indicated for patients aged ≥12 years with obesity or adults with overweight and the presence of one or more weight-related health conditions. Semaglutide (Wegovy) is also indicated for CV risk reduction in adults with established CVD and either obesity or overweight.24
Semaglutide titrated to 2.4 mg weekly was evaluated in a 68-week, randomized, double-blind study.27 Adults aged ≥18 years (N = 611) with BMI ≥30 kg/m 2 or BMI ≥27 kg/m 2 plus one concurrent weight-related condition participated in this phase IIIa study. Semaglutide resulted in a significantly higher weight change from baseline than placebo (mean difference -10.3%; 95% CI, -12.0 to -8.6; P <.0001). In addition, significantly more participants achieved 5% and 10% reduction in body weight with semaglutide compared with placebo (86.6% vs. 47.6% and 75.3% vs. 27%, respectively; P <.001).27
A second 68-week trial evaluated weekly semaglutide 2.4 mg versus placebo in adults aged ≥18 years without diabetes (N = 1,961) with BMI ≥30 kg/m 2 or BMI ≥27 kg/m 2 plus one or more concurrent conditions.28 Significantly more weight reduction was achieved with semaglutide 2.4 mg weekly (-14.9%) than placebo (-2.4%; 95% CI, -13.4 to -11.5; P <.002). The likelihood of achieving 5% to 15% or more weight reduction compared with baseline body weight was also higher in the semaglutide group (P <.001).28
The STEP 8 trial evaluated weekly semaglutide 2.4 mg versus daily liraglutide 3 mg in adults aged ≥18 years without diabetes (N = 338) with BMI ≥30 kg/m 2 or BMI ≥27 kg/m 2 with one or more weight-related conditions.29 Participants were randomized 3:1:3:1 to semaglutide:placebo (n = 126) or liraglutide:placebo (n = 127). Baseline participant characteristics were a mean age of 49 years, 78.4% female, mean body weight 104.5 kg, and mean BMI 37.5 kg/m 2 . The semaglutide group achieved significantly higher weight reduction from baseline (-15.8%) compared with the liraglutide group (-6.4%) at 68 weeks (95% CI, -12.0 to -6.8; P <.001). The percentage of semaglutide participants achieving 10%, 15%, or 20% weight reduction compared with baseline was also significantly higher compared with liraglutide (70.9% to 38.5% vs. 25.6% to 6%, respectively; P <.001).29
GLP-1 RA and GIP Combination
Glucose-dependent insulinotropic polypeptide (GIP) is released from K-cells in the duodenum and jejunum in response to intake of high-fat foods and carbohydrates.21 In addition to the actions of GLP-1, GIP also potentiates glucose-stimulated insulin secretion. Like GLP-1 receptors, hypothalamic expression of GIP receptors also modulates energy balance and nutrient intake.21,30 GIP receptors affect lipid metabolism, increase lipid storage, and improve adipocyte insulin sensitivity.30
One GLP-1 RA/GIP combination medication, tirzepatide (Zepbound), was approved by the FDA for weight loss in 2022.31 Tirzepatide was initially approved for use to improve glycemic control in T2DM under the brand-name Mounjaro.32 Dosing and administration options for both tirzepatide forms are similar. Tirzepatide (Zepbound) may be used for adults with BMI ≥30 kg/m 2 or BMI ≥27 kg/ m 2 with at least one weight-related comorbid health condition (see TABLE 3).31
Significantly greater weight reduction with tirzepatide was found in a 72-week, phase III, doubleblind, randomized, placebo-controlled trial (SURMOUNT-1) evaluating adults aged ≥18 years without diabetes with BMI ≥30 kg/m 2 or BMI ≥27 kg/m 2 and at least one weight-related health condition.33 Participants (N = 2,539) were randomized to tirzepatide 5 mg, 10 mg, or 15 mg or placebo. At baseline, 94% participants had a BMI ≥30 kg/m 2 with mean body weight of 104.8 kg. At Week 72, mean percent body weight reduction was significantly higher (P <.001) in the 5-mg, 10-mg, and 15-mg tirzepatide groups (-15.0%, -19.5%, and -20.9%, respectively) compared with placebo (-3.9%). Weight loss ≥5% from baseline occurred in 85% to 91% of tirzepatide participants versus 35% on placebo (P <.001). A 20% weight reduction from baseline was achieved by 50% and 57% of tirzepatide 10-mg and 15-mg groups compared with 3% in the placebo group (P <.001).33
SURMOUNT-4 was a two-part, phase III, randomized, open-label withdrawal trial that evaluated adults aged ≥18 years with BMI ≥30 kg/m 2 or BMI ≥27 kg/m 2 plus one weight-related comorbidity.34 In part one, participants (n = 783) were titrated to tirzepatide 10 mg or 15 mg weekly for 36 weeks. In part two, participants (n = 670) were then randomized to continue tirzepatide (n = 335) or switch to placebo (n = 335) for the next 52 weeks. From Weeks 36 to 88, the tirzepatide group achieved -5.5% weight reduction from baseline versus an increase of 14% in the placebo group (mean difference -19.4%; 95% CI, -21.2% to -17.7%; P <.001). Significantly more tirzepatide participants at Week 88 had maintained 80% weight loss achieved during the lead-in period (89.5% vs. 16.6%, P <.001).34
CV Risk Reduction
Among the GLP-1 RA and GLP-1 RA/GIP medica- tions for weight loss, semaglutide (Wegovy) is the only GLP-1 RA with a CV risk reduction indication in patients with established CVD and either obesity or overweight. 22 However, studies also support CV risk reduction in individuals without diabetes using tirzepatide for weight loss.35
The SELECT trial demonstrated a decrease in CV-related events and CV-related death in individuals without diabetes aged ≥45 years with pre-existing CVD and BMI ≥27 kg/m 2 when treated with semaglutide 2.4 mg weekly.35 This time-to-first-event superiority trial randomized participants to placebo (n = 8,801) or semaglutide 2.4 mg weekly (n = 8,803). After completing a mean therapy duration of 34.2 months and a mean follow-up of 39.8 months, a primary CV endpoint event (CV death, nonfatal myocardial infarction, or stroke) occurred in 6.5% of the semaglutide group and 8.0% of the placebo group (hazard ratio, 0.80; 95% CI, 0.72-0.90; P <.001).35
Post hoc analysis of the tirzepatide versus placebo SURMOUNT-1 trial evaluated predicted 10-year atherosclerotic CVD (ASCVD) scores at baseline, Week 24, and Week 72 in participants without ASCVD history (N = 2,461).33,36 Investigators found that from baseline to Week 72, ASCVD risk increased in placebo patients (12%) and decreased in the tirzepatide groups (-23.5% to –16.4%; P <.001).36
Side Effects and Contraindications
The most common side effects noted with GLP-1 RA and GLP-1 RA/GIP use are often GI in nature. Nausea, vomiting, diarrhea, and constipation occur in ≥10% and dyspepsia and abdominal pain in ≥5% of individuals using GLP-1 RA or GLP-1 RA/GIP medications.23,24,31 In the STEP 8 trial, GI side effects occurred in 84.1% of semaglutide participants and 82.7% of liraglutide participants.29 GI side effects typically occur more frequently at higher doses and are often mitigated with dose reduction or discontinuation of the medication.23,24,31 Studies evaluating long-term adherence to GLP-1 RA or GLP-1 RA/GIP therapy have found that up to 50% of individuals stop therapy by 12 months. 37 While GI side effects are one potential factor, more studies are needed regarding why adherence decreases within the first 12 months.
Semaglutide, liraglutide, and tirzepatide each contain warnings regarding the potential for acute pancreatitis and acute gallbladder disease.23,24,31 Acute pancreatitis is considered a class effect for GLP-1 RAs. With semaglutide (Wegovy), pancreatitis occurred at a higher rate (0.2 cases per 100 patient-years) than placebo (0.1 cases per 100 patient-years).24 Cholelithiasis and cholecystitis were also reported at higher rates in semaglutide groups (1.6% and 0.6%) compared with placebo (0.7% and 0.2%). Risk of cholelithiasis or cholecystitis is higher in pediatric patients treated with semaglutide (3.8% and 0.8% vs. 0% placebo).24 Periodic monitoring of pulse is also recommended due to potential for increased heart rate with these agents. While no renal or hepatic dosing modifications are needed, monitoring for changes in volume status and electrolytes is important to reduce the risk of acute kidney injury if GI side effects occur.23,24,31
GLP-1 RAs and GLP-1 RA/GIPs are contraindicated in pregnancy and should be discontinued if pregnancy occurs. Because semaglutide has a long half-life, it should be stopped at least 2 months before the patient plans to become pregnant. No data currently exist regarding presence of liraglutide, semaglutide, or tirzepatide in breast milk; therefore, a risk-versus-benefit discussion should occur prior to initiation of a GLP-1 RA or GLP-1 RA/GIP during lactation.23,24,31
Tirzepatide can cause changes to concentrations of oral hormonal contraceptives due to delays in gastric emptying.31 Because GLP-1 RAs and GLP-1 RA/GIPs slow transit rates through the GI tract, monitoring of medications with narrow therapeutic indices is recommended.23,24,31
Delayed gastric emptying caused by GLP-1 RA and GLP-1 RA/GIP led the American Society of Anesthesiologists to release guidelines for surgical procedures and timing of administration of a GLP-1 RA and GLP-1 RA/GIP to reduce potential for regurgitation and pulmonary aspiration of stomach contents while under sedation or anesthesia.38 These guidelines recommend holding liraglutide (and other daily GLP-1 RAs) on the day of the procedure. Holding weekly semaglutide (and other weekly GLP-1 RAs) and weekly tirzepatide at least 1 week prior to surgery/procedure is recommended.38
Use of GLP-1 RAs and GLP-1 RA/GIPs is not recommended in patients with gastroparesis due to already delayed gastric emptying and slowed GI transit time.23,24,31 Little data exist regarding use of GLP-1 RAs and GLP-1 RA/GIPs in this patient population.
Black Box Warning
GLP-1 RAs and GLP-1 RA/GIPs have a black box warning regarding potential risk of thyroid C-cell tumors and medullary thyroid carcinoma (MTC) in humans. Use of GLP-1 RAs and GLP-1 RA/GIPs in patients with history of multiple endocrine neoplasia syndrome type 2 is also contraindicated due to concern for risk of MTC and thyroid tumors.23,24,31
A systematic review and meta-analysis of 26 trials lasting at least 52 weeks and reporting at least one incident case of thyroid cancer found the risk of overall thyroid cancer significantly increased with GLP-1 RA therapy (odds ratio, 1.52; 95% CI, 1.01-2.29; P = .04; I2 = 0%) and a number needed to harm of 1,349 at 5 years. Medullary and papillary thyroid cancers were not significantly associated with GLP-1 RA use39
In addition, GLP-1 RA use increased potential development of thyroid neoplasms and hyperplasia in patients with diabetes when compared with monotherapy with sodium-glucose cotransporter-2 (SGLT-2) inhibitors.40 This analysis included >17,653 reports made to the FDA Adverse Event Reporting System database over 12 years regarding GLP-1 RA monotherapy (n semaglutide = 3,230; n liraglutide = 6,162). Individual reporting odds ratios were significantly higher with semaglutide (20.78; 95% CI, 9.19-47.03) and liraglutide (35.92; 95% CI, 16.71-77.20) com- pared with SGLT-2 inhibitors.40
Thyroid gland dysfunction can occur with metabolic health conditions such as obesity and diabetes. Thyroid monitoring during GLP-1 RA and GLP-1 RA/GIP therapy, whether as monotherapy or concurrent drug therapy, is recommended. Due to the black box warning, a thorough screening of individual and family history of thyroid function and/or cancer is warranted before starting these agents.
ADDITIONAL CONSIDERATIONS
The estimated cost of weight loss-specific forms of semaglutide, liraglutide, and tirzepatide ranges from $1,000 to $1,400 per 28-day supply at the time of this writing.41 Insurance coverage is variable and dependent on the plan's formulary and prior authorization requirements. Patient-assistance programs may be available.
Drug shortages also challenge accessibility. Shortages of these medications began in 2022 and have persisted intermittently into 2024. The shortages are not specific to any one type or strength of GLP-1 RA or GLP1 RA/GIP, and any may be on shortage at any given time. Strategies for addressing shortages have included interchanging the prescribed GLP-1 RA with an alternative GLP-1 RA or GLP-1 RA/GIP and use of alternative dosing schedules or alternative agents.42
In addition to improving accessibility, patients may switch from one agent to another due to adherence concerns (daily vs. weekly administration), cost/cover-age (changes in insurance formularies), or mitigating adverse effects. An algorithm for switching between a daily and weekly GLP-1 RA has been proposed but does not currently include tirzepatide or weight-loss doses of liraglutide and semaglutide.43
Compounded formulations of liraglutide, semaglutide, and tirzepatide have been used by patients and providers to alleviate cost/coverage and accessibility issues. In 2023, the Obesity Medicine Association released a position statement and a follow-up "frequently asked questions (FAQ)" to address compounded polypeptides.44,45 The position statement defined compounding of GLP-1 RAs and GLP-1/GIPs and how it might provide GLP-1 RAs or GLP-1 RA/GIPs to patients under the provider/ patient/pharmacist/compounder relationship.43 The FAQ statement provided further detail regarding the compounding process, FDA requirements, the importance of monitoring for and avoiding counterfeit products, and how compounding may fit into the drug shortage process.45 The Obesity Medicine Association emphasized obtaining compounded products from reputable sources, following guidelines and good manufacturing practices, and maintaining the provider/patient/pharmacist/compounder relationship.44,45
Case reports of 10- to 20-fold dosing errors with compounded semaglutide formulations have been summarized.46 The FDA released an alert regarding dosing errors of compounded injectable semaglutide products to healthcare providers, compounders, and patients.47 This alert identified errors related to incorrect measurement of dose prior to administration, resulting in higher doses administered than prescribed or recommended. The confusion between units and volume resulted in larger than recommended doses of semaglutide to be administered as a first dose, for example, administration of 1 mL (2.5 mg) of a 2.5-mg/1-mL solution of semaglutide instead of 0.1 mL (0.25 mg). Patients reported significant GI side effects such as vomiting and decreased appetite from the errors. 46 Overdose with compounded GLP-1 RA and GLP-1 RA/GIP products remains a concern.
ROLE OF THE PHARMACIST
Abundant opportunities exist for pharmacist involvement in obesity and weight management. The complexity of obesity management involves pharmacotherapy and addressing lifestyle changes and psychosocial and accessibility barriers. While more robust studies are needed, the value of pharmacist engagement in obesity management has been demonstrated.48 Pharmacists can assist with medication selection and optimization to reduce potential side effects and treatment burden and to improve adherence. Pharmacists may also monitor for efficacy, side effects, renal/hepatic function, lipids, vital signs (blood pressure and heart rate), reduction of weight gain-causing medications, and assessment of CV risk or past history of CVD. Exploring methods to reduce cost and improve coverage of weight-loss medications is important, such as assisting with the prior authorization process, educating regarding potential assistance programs, and recommending alternative agents. Education on device use and appropriate sharps disposal (where applicable) is important for patients, caregivers, and providers. Mitigating challenges to accessibility due to drug shortages is necessary. Pharmacists should educate providers and patients and recommend guideline-based alternatives that are cost-effective and achieve weight-loss goals without increasing side effects, reducing adherence, or increasing treatment burden.
CONCLUSION
The landscape of obesity management is evolving at a rapid rate. Oral and/or injectable pharmacotherapy options may be used in conjunction with lifestyle modifications and behavior change. Education of providers and patients regarding new and emerging therapies and how these fit into current guidelines and treatment and prescribing parameters is imperative.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
- Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960-1962 through 2017-2018. CDC. www.cdc.gov/nchs/data/hestat/obesity-adult-17-18/obesity-adult.htm. Accessed September 21, 2024.
- CDC. Adult obesity facts. www.cdc.gov/obesity/adult-obesity-facts. Accessed September 21, 2024.
- WHO. Obesity and overweight. March 1, 2024. www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed Septem- ber 21, 2024.
- American Diabetes Association Professional Practice Committee. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47(Suppl 1):s145-s157.
- Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(Suppl 3):1-203.
- Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192(31):e875-e891.
- Department of Veterans Affairs. VA/DoD clinical practice guideline for the management of adult overweight and obesity. www.healthquality.va.gov/guidelines/CD/obesity/VADoDObesityCPGFinal5087242020. pdf. Accessed September 11, 2024.
- Yurista SR, Eder RA, Feeley M, et al. A closer look at ACC/AHA and ESC guidelines for managing obesity and overweight in adults. JACC Adv. 2023;2(7):100570.
- Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice (published correction appears in Eur Heart J. 2022;43[42]:4468). Eur Heart J. 2021;42(34):3227-3337.
- Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102-2107.
- Sheehan A, Chen JT, Yanovski JA. Obesity. In: DiPiro JT, Yee GC, Haines ST, et al, eds. DiPiro's Pharmacotherapy: A Pathophysiologic Approach. 12th ed. New York, NY: McGraw Hill; 2023.
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines (published correction appears in J Am Coll Cardiol. 2020;75[7]:840). J Am Coll Cardiol. 2019;74(10):e177-e232.
- Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society clinical practice guideline (published correction appears in J Clin Endocrinol Metab. 2015;100(5):2135-2136). J Clin Endocrinol Metab. 2015;100(2):342- 362.
- Miller SJ, Brown S. Overweight and obesity. In: Krinsky DL, Ferreri SP, Hemstreet BA, et al, eds. Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care. 21st ed. Washington, DC: American Pharmacists Association; 2024:547-566.
- Maunder A, Bessell E, Lauche R, et al. Effectiveness of herbal medicines for weight loss: a systematic review and meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2020;22(6):891- 903.
- FDA. Information on select dietary supplement ingredients and other substances. www.fda.gov/food/dietary-supplements/information-select-dietary-supplement-ingredients-and-other-substances. Accessed September 21, 2024.
- Liraglutide: drug information. LexiDrugs. UpToDate Lexidrug. http://online.lexi.com. Accessed September 11, 2024. 18. Imcivree (setmelanotide) product information. Boston, MA: Rhythm Pharmaceuticals, Inc; June 2022.
- Imcivree (setmelanotide) product information. Boston, MA: Rhythm Pharmaceuticals, Inc; June 2022.
- FDA. FDA drug safety communication: FDA recommends against the continued use of Meridia (sibutramine). October 8, 2010. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-recommends-against-continued-use-meridia-sibutramine. Accessed September 20, 2024.
- FDA. FDA requests the withdrawal of the weight-loss drug Belviq, Belviq XR (lorcaserin) from the market. February 13, 2020. www.fda.gov/drugs/drug-safety-and-availability/fda-requests-withdrawal-weight-loss-drug-belviq-belviq-xr-lorcaserin-market. Accessed September 20, 2024.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132;(7):2131-2157.
- Liraglutide (injection) product information. Parsippany, NJ: Teva Pharmaceuticals; January 2024.
- Saxenda (liraglutide injection) product information. Plainsboro, NJ: Novo Nordisk, Inc; May 2023.
- Wegovy (semaglutide injection) product information. Plainsboro, NJ: Novo Nordisk, Inc; March 2024.
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11-22.
- le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight manage- ment in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389(10077):1399-1409.
- Wadden TA, Bailey TS, Billings LK, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403-1413.
- Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002.
- Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes. JAMA. 2022;327(2):138- 150.
- Lafferty RA, Flatt PR, Irwin N. GLP-1/GIP analogs: potential impact in the landscape of obesity pharmacotherapy. Expert Opin Pharmacother. 2023;24(5):587-597.
- Zepbound (tirzepatide injection, solution) product information. Indianapolis, IN: Eli Lilly and Company; March 2024.
- Mounjaro (tirzepatide injection, solution) product information. Indianapolis, IN: Eli Lilly and Company; February 2024.
- Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205- 216.
- Aronne LJ, Sattar N, Horn DB, et al. Continued treatment with tirzepatide for maintenance of weight reduction in adults with obesity: the SURMOUNT-4 randomized clinical trial. JAMA. 2024;331(1):38- 48.
- Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389(24):2221-2232.
- Hankosky ER, Wang H, Neff LM, et al. Tirzepatide reduces the predicted risk of atherosclerotic cardiovascular disease and improves cardiometabolic risk factors in adults with obesity or overweight: SURMOUNT-1 post hoc analysis. Diabetes Obes Metab. 2024;26(1):319- 328.
- Weiss T, Carr RD, Pal S, et al. Real-world adherence and discon- tinuation of glucagon-like peptide-1 receptor agonists therapy in type 2 diabetes mellitus patients in the United States. Patient Prefer Adherence. 2020;14:2337-2345.
- Ushakumari DS, Sladen RN. ASA Consensus-based guidance on preoperative management of patients on glucagon-like peptide-1 receptor agonists. Anesthesiology. 2024;140(2):346-348.
- Silverri GA, Monami M, Gallo M, et al. Glucagon-like peptide-1 receptor agonists and risk of thyroid cancer: a systematic review and meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2024;26:891-900
- Makunts T, Joulfayan H, Abagyan R. Thyroid hyperplasia and neoplasm adverse events associated with glucagon-like peptide-1 recep- tor agonists in the Food and Drug Administration adverse event reporting system: retrospective analysis. JMIRx Med. 2024;5:e55976.
- Clinical resource, comparison of GLP-1 agonists. Pharmacist's Letter/Pharmacy Technician's Letter/Prescriber Insights. August 2024.
- Whitley HP, Trujillo JM, Neumiller JJ. Special report: potential strategies for addressing GLP-1 and dual GLP-1/GIP receptor agonist shortages. Clin Diabetes. 2023;41(3):467-473.
- Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon-like peptide-1 receptor agonists: rational and practical guidance. Clin Diab J. 2020;38(4):390-402.
- Fitch A, Auriemma A, Bays HE. Compounded peptides: an Obe- sity Medicine Association position statement. Obes Pillars. 2023;6:100061.
- Bays HE, Fitch A, Fancavilla Brown C, et al. Frequently asked questions to the 2023 Obesity Medicine Association position statement on compounded peptides: a call for action. Obes Pillars. 2024;11:100122.
- Lambson JE, Flegal SC, Johnson AR. Administration errors of compounded semaglutide reported to a poison control center—case series. J Am Pharm Assoc. 2023;63:1643-1645.
- FDA. FDA alerts health care providers, compounders and patients of dosing errors associated with compounded injectable semaglutide products. www.fda.gov/drugs/human-drug-compounding/fda-alerts-health-care-providers-compounders-and-patients-dosing-errors-associated-compounded. Accessed September 24, 2024.
- Clements JN, Albanese NP, D'Souza JJ, et al. Clinical review and role of clinical pharmacists in obesity management: an opinion of the endocrine and metabolism practice and research network of the American College of Clinical Pharmacy. J Am Coll Clin Pharm. 2021;4(11):1469-1484.