Optimizing Heart Failure Management in Women
RELEASE DATE
September 1, 2025
EXPIRATION DATE
September 30, 2027
FACULTY
Meg Conger Ason, PharmD, BCACP, CPP
Clinical Pharmacist
Atrium Health
Huntersville, North Carolina
Emily Gwynn, PharmD
Clinical Pharmacist
Atrium Health
Charlotte, North Carolina
Jennifer LaPreze, PharmD, BCACP, CDCES, CPP
Clinical Pharmacist Specialist
Atrium Health
Charlotte, North Carolina
DISCLOSURE STATEMENTS
Drs. Conger Ason, Gwynn, and LaPreze have no actual or potential conflicts of interest in relation to this activity.
Postgraduate Healthcare Education, LLC does not view the existence of relationships as an implication of bias or that the value of the material is decreased. The content of the activity was planned to be balanced, objective, and scientifically rigorous. Occasionally, authors may express opinions that represent their own viewpoint. Conclusions drawn by participants should be derived from objective analysis of scientific data.
ACCREDITATION STATEMENT
 Pharmacy
Pharmacy
Postgraduate Healthcare Education, LLC is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
UAN: 0430-0000-25-090-H01-P
Exam processing and other inquiries to:
CE Customer Service: (800) 825-4696 or cecustomerservice@powerpak.com
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
GOAL
To explain the nuances of heart failure pathophysiology and management in women.
OBJECTIVES
After completing this activity, the participant should be able to:
- Explain physiological differences in heart failure between men and women.
- Identify differences in medication treatment regimens for heart failure management.
- Describe pathophysiology and management of heart failure in pregnant and nonpregnant individuals.
- Recognize the role of the pharmacist in heart failure management.
ABSTRACT: Heart failure (HF) impacts millions of Americans each year and is expected to continue to grow. Women constitute roughly one-half of patients with HF; however, they can exhibit different pathophysiology and symptoms. They are more prone to developing HF with preserved ejection fraction over other types of HF. In addition, cardiac disease related to pregnancy is a type of HF that exclusively impacts women. Because women are less likely to be included in HF trials, more investigation is needed into how to optimally manage these unique patients.
Heart failure (HF) in the United States is a growing cause of morbidity, mortality, and healthcare expense, with the Heart Failure Society of America reporting that more than 6.7 million Americans aged older than 20 years had the condition in 2024.1 The number of people impacted by HF is expected to grow to 8.7 million by 2030 and more than 11 million by 2050.2 In 2021, approximately 2.6 million HF patients in the U.S. were cisgendered women.3
There are significant differences in epidemiology, clinical manifestations, and outcomes between men and women with HF. To clarify, the term “women” in this article will mean cisgendered women. There is little to no information about the impact of gender identity in HF studies.4
Although recent treatment guidelines note differences between ethnic and racial groups, recommendations are not sex-based.5 In fact, many of the differences noted between the sexes have been gleaned from observational cohort studies, such as the Framingham Heart Study, and other analyses of health systems data.6
Overall, women also tend to be underrepresented in clinical trials, with enrollment limited to 20% to 25% of the study population.6 This can limit the generalizability of guidelines.6 Studies are also not routinely powered to evaluate differences between sexes.4
DIAGNOSIS AND CLASSIFICATION
HF is defined by the American Heart Association/American College of Cardiology/Heart Failure Society of America 2022 guidelines as a “complex clinical syndrome with symptoms and signs that result from any structural or functional impairment of ventricular filling or ejection of blood.”5 Criteria for HF diagnosis currently are sex neutral, based on left ventricular ejection fraction (EF). HF with reduced EF (HFrEF) is defined as EF ≤40%. Patients with HF with mildly reduced EF have an EF from 41% to 49%, and patients with HF with preserved EF (HFpEF) have an EF ≥50%.5 Some clinical studies have set EF thresholds for patient inclusion at less than 40%, 45%, or 50%, which can also reduce generalizability.3
HF is classified into four stages (A through D) that include persons at risk; asymptomatic persons with structural heart disease or evidence of increased filling pressures or abnormal laboratory result; symptomatic HF; and advanced HF (as shown in TABLE 1).5 Symptomatic HF (stage C) is subdivided into the New York Heart Association (NYHA) classifications based on severity of symptoms and functional status.5
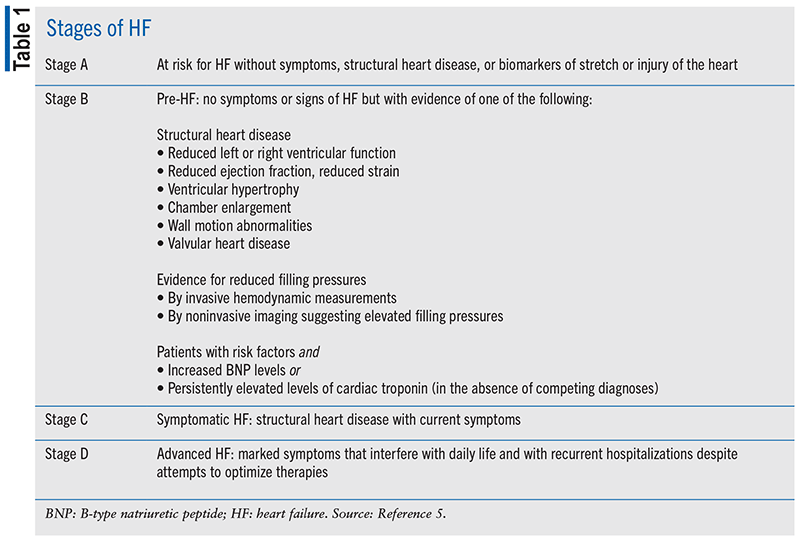
Even though the NYHA classifications are subjective and can change over time, guidelines recommend using them to help determine a symptomatic HF patient’s status in the disease course. The guidelines divide the trajectory of the disease into four phases: new onset; resolution of symptoms, meaning remission of symptoms and previous structural or functional disease; persistent HF with ongoing symptoms and/or limited functional capacity; and worsening HF.5
HF DIFFERENCES BETWEEN SEXES
Overall, the risk of developing HF over the course of a person’s life is about 20% in both sexes.7 Women tend to present with new diagnoses of HF at older ages. For example, as men age, the incidence of HF doubles every 10 years between the ages of 65 and 85 years. In women, the incidence triples.8
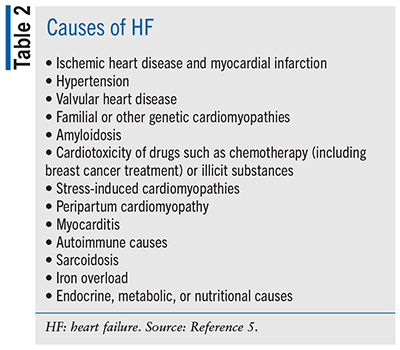
In addition to age, other risk factors for HF include hypertension, cardiovascular disease, obesity, diabetes, exposure to cardiotoxic agents, genetic variant for cardiomyopathy, or family history of cardiomyopathy.5 Modifiable risk factors such as obesity, hypertension, diabetes, and smoking also contribute to the possibility of developing HF, particularly in younger patients.1 See TABLE 2 for other causes of HF.
Both men and women are at risk for mortality, hospitalization, and poor quality of life from HF, but men have a higher mortality rate.4 In 2019, the age-adjusted mortality rate of men with HF was 70.4 per 100,000 persons; in women, it was 40.9 per 100,000 persons.4 Women with HF tend to survive longer than men, no matter the cause of their disease.8 Women’s longevity is particularly apparent for female patients whose HF is not related to cardiac ischemia.2
Hospitalizations increased from just over 1 million in 2008 to almost 1.3 million in 2018, particularly in members of underrepresented racial and ethnic groups, according to the U.S. National Inpatient Sample database.1 In an analysis of data from 2002 to 2016, there was a decrease in mortality during hospitalization, from 6.8% to 4.9%.1 The data also showed a decrease in length of stay and an increase in the number of patients discharged to a long-term care facility.1
Symptoms of HF are typically similar in men and women; however, women tend to report more edema, dyspnea on exertion, and difficulty exercising.8 They also have worse quality-of-life scores than men, despite controlling for age, EF, and NYHA classification.8 It is theorized that women tend to be more symptomatic because of greater diastolic dysfunction.8
Important distinctions between the sexes are also evident in the different subtypes of HF.1 For example, a greater proportion of overall hospitalizations for HFrEF were in men (60.5%), and a greater number of hospitalizations for HFpEF were in women (62.5%).1
HFrEF
Women have lower a lifetime risk of developing HFrEF, 5.8% compared with 10.6% in men, based on two prospective cohort studies, the Cardiovascular Health Study and the Multi-Ethnic Study of Atherosclerosis.3 Risk factors for HFrEF are male sex, previous myocardial infarction, electrocardiographic left ventricular hypertrophy, and left bundle-branch block.1
Women hospitalized with HFrEF tend to be older and more likely to have underlying conditions such as hypertension and valvular disease. They are less likely to be smokers or to have peripheral vascular disease. Women with reduced EF also tend to have poorer quality of life, despite lower mortality rates, as shown in the PARADIGM- HF and ATMOSPHERE trials.3
Sex differences in cardiac aging help explain why fewer women develop HFrEF.3 Fewer women tend to have obstructive coronary artery disease, and they also tend to have less apoptosis and necrosis of myocytes and less remodeling after a heart attack.7
Recent trends have shown a decrease in HFrEF in women but an increase in HFpEF, particularly in black women.4
HFpEF
Women have a 2.8-fold increased risk of having HFpEF compared with men, based on an analysis of the multidecade, population-based Framingham Heart Study.9 Older age, higher systolic blood pressure, and higher BMI were independent predictors of HFpEF. Other risk factors noted in Framingham are smoking and history of atrial fibrillation. These analyses have not shown that female sex is a predictor of HFpEF; however, they suggest that the links are more strongly related to aging.3
Physiologic characteristics of women’s hearts and vasculature make them more likely to develop HFpEF. Female patients have increased diastolic, systolic, and arterial elastance and smaller diameter of vasculature. In addition to greater concentric remodeling, they tend to have more impairment of left ventricular relaxation.3
Obesity also has an important link to HFpEF, and it is more apparent in sex-stratified analyses of several studies, including Framingham. Analyses of the Women’s Health Initiative showed a stronger link between obesity and HFpEF than HFrEF in women.3 It is theorized that this link is related to systemic inflammatory conditions caused by metabolic syndrome, obesity, and diabetes.3
Diabetes is more likely to be a more contributory risk factor for women in HFpEF, as it tends to be linked to concentric remodeling, which is linked to HFpEF.7
Other researchers postulate that estrogen’s protective qualities over the vasculature may make postmenopausal women more vulnerable to development of HFpEF.3
TREATMENT OF HF
Proper management of HF is imperative to improve patient outcomes and quality of life. This article will focus on medical therapy and defer discussion of devices and other treatment modalities. Additionally, this article focuses on management of chronic HF and not acute HF exacerbation management.
Guideline-directed medical therapy (GDMT) has shown a reduction in both mortality and morbidity within 30 days of starting treatment.10 Titration of GDMT to target dose or maximally tolerated dose is recommended within 3 to 6 months.11 However, treatments that improve survival are underused in HF, and age- and population-adjusted mortality in the U.S. have continued to rise over time, reaching 21.0 per 100,000 people in 2022.1
HFrEF Medication Therapy
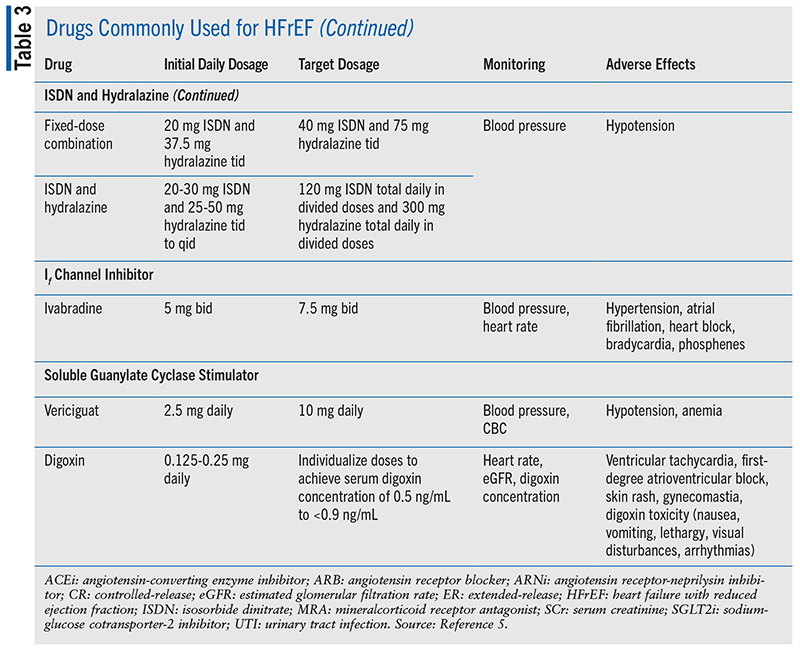
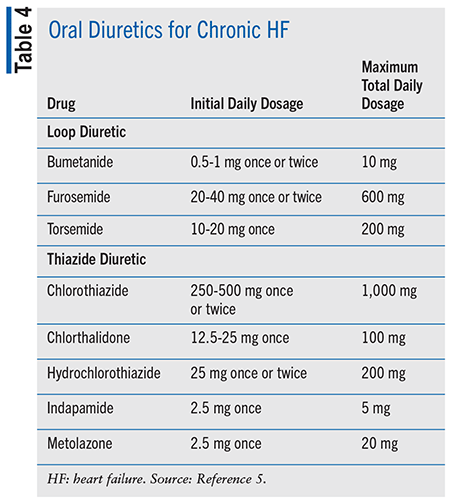
The four pillars of medication classes for HFrEF treatment that are considered a Class 1 recommendation are renin-angiotensin system (RAAS) inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRAs), and sodium glucose cotransporter-2 inhibitors (SGLT2is).5 TABLE 3 shows initial and target doses for these medications along with additional treatment options and necessary monitoring for potential adverse events.5 Diuretics are recommended in patients for volume management as needed and are included in TABLE 4.5 Loop diuretics are preferred in most patients with HF, and thiazide diuretics may be considered in patients with hypertension and HF with fluid retention.5 It is estimated that a 70-year-old patient with HFrEF will gain 5 years of life expectancy if she is treated with the quadruple therapy of RAAS inhibitors, beta-blockers, MRAs, and SGLT2is.12
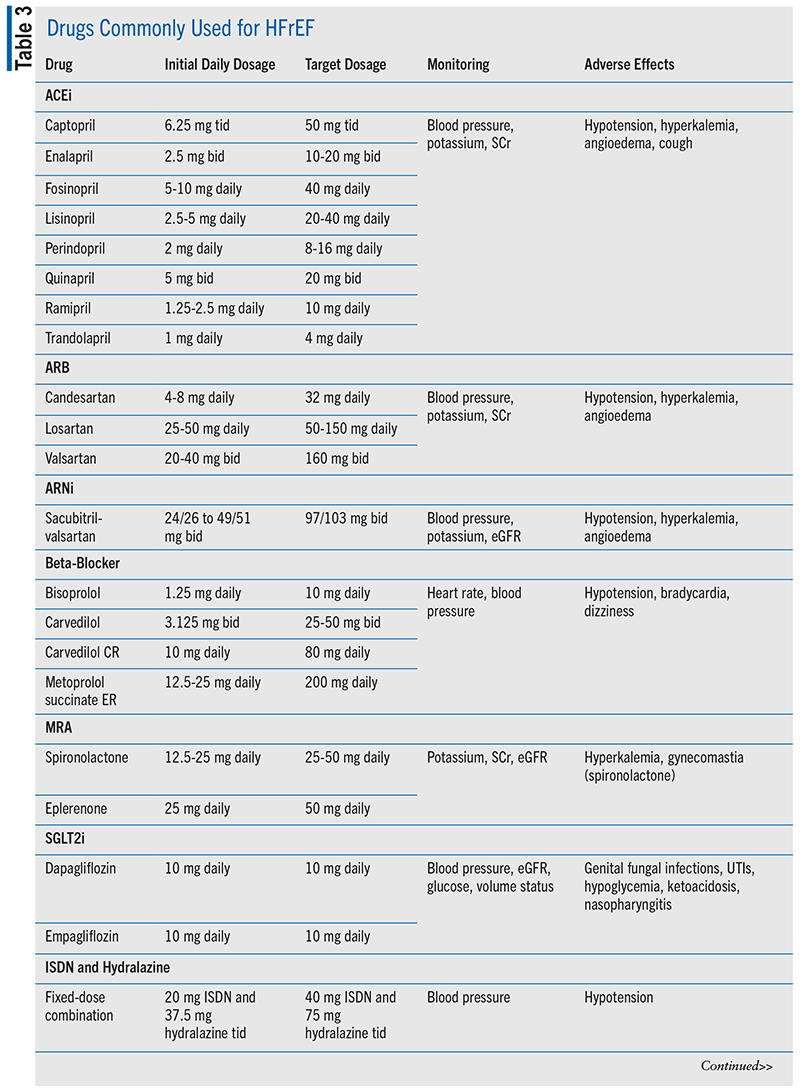
RAAS Inhibition: Inhibition of RAAS with an angiotensin receptor/neprilysin inhibitor (ARNi), an angiotensin-converting enzyme inhibitor (ACEi), or an angiotensin receptor blocker (ARB) is recommended to reduce morbidity and mortality in patients with HFrEF.5 ACEis have been shown to reduce morbidity and mortality in HFrEF.5 ARBs have been shown to result in a decrease in mortality and HF hospitalizations in patients with HFrEF.5 ARBs may be considered as an alternative for patients who are experiencing cough with ACEis or in patients with a history of angioedema as an alternative to ACEi and ARNi.5,13 To improve morbidity and mortality, patients with chronic symptomatic HFrEF with NYHA Class II or III symptoms who tolerate an ACEi or ARB should be switched to an ARNi.5 If individuals are switched from an ACEi to an ARNi, at least a 36-hour washout period is needed owing to the risk of life-threatening angioedema.13
Beta-Blockers: In clinical studies of patients with HFrEF, only three beta-blockers (bisoprolol, carvedilol, and sustained-release metoprolol succinate) have reduced the risk of death and the combined risk of death or hospitalization.5 These beta-blockers can improve left ventricular EF (LVEF) and lessen the symptoms of HF and are recommended in all patients who are diagnosed with HFrEF unless they are not tolerated by the patient or are contraindicated for therapy.5 Contraindications include critical limb ischemia, severe asthma or chronic obstructive pulmonary disease (COPD), recent exacerbation of HF, or second- or third-degree atrioventricular blocks. This is not an overall class effect. Beta-blockers have different selectivity that is categorized as nonselective with similar beta-1 and beta-2 activity (these are not used for HFrEF); beta-1 selective with higher affinity for beta-1-adrenoreceptors (metoprolol, bisoprolol) or beta-blockers with additional alpha-1-adrenoreceptor antagonism; and peripheral vasodilation (carvedilol).5 Metoprolol and bisoprolol are preferred in patients with mild asthma or COPD, while carvedilol is preferred in hypertension or peripheral artery disease.5 When a beta-blocker is being selected, treatment should be individualized based on other comorbidities. Abrupt withdrawal of beta-blocker therapy can cause clinical deterioration and should be avoided.5
MRAs: In clinical studies, patients with HFrEF treated with MRAs had reductions in sudden cardiac death, HF hospitalizations, and all-cause mortality. MRAs are contraindicated at initiation if serum potassium is >5.0 mEq/L or estimated glomerular filtration rate (eGFR) is <30 mL/min/1.73 m2.5
SGLT2is: SGLT2is are recommended to reduce hospitalization for HF and cardiovascular mortality in individuals with symptomatic chronic HFrEF without regard to diagnosis of type 2 diabetes.5 In the DAPA-HF and EMPEROR-Reduced trials, SGLT2is reduced HF hospitalization or cardiovascular death by 25%.5
Additional Medical Therapies
Additional medical therapies may be considered once GDMT is optimized.
Hydralazine and Isosorbide Dinitrate: Hydralazine and isosorbide dinitrate have been shown to reduce morbidity and mortality in patients who self-identified as African American with NYHA III and IV symptomatic HFrEF despite receiving optimal therapy with RAAS, beta-blockers, MRAs, and SGLT2is.5 It is unclear if a benefit exists for non–African Americans with HFrEF. Currently, there are not enough data available about dual therapy of hydralazine and isosorbide dinitrate with ARNi. In individuals who are not able to tolerate therapies including ARNi, ACEi, or ARB owing to renal insufficiency or drug intolerance, hydralazine and isosorbide dinitrate may be considered to reduce morbidity and mortality.These individuals should be referred to an HF specialist for evaluation.5
Ivabradine: Ivabradine can be considered in symptomatic patients with stable chronic HFrEF who are taking the maximum tolerated doses of beta-blockers and who are in sinus rhythm with a heart rate >70 beats/min at rest. The SHIFT trial showed that ivabradine reduced cardiovascular death or HF hospitalizations in selected patients. Ivabradine increases the risk of atrial fibrillation and should be discontinued if it develops. It is contraindicated in patients with acute decompensated HF, hypotension (blood pressure <90/50 mmHg), bradycardia (heart rate <60 bpm prior to treatment), or sick sinus syndrome, sinoatrial block, or third-degree atrioventricular block unless a functioning demand pacemaker is present.5 Ivabradine is primarily metabolized by CYP3A4, and concomitant use of strong CYP3A4 inhibitors is contraindicated.
Vericiguat: Patients who are at high risk with recent worsening of HF and are already on GDMT may benefit from vericiguat. Vericiguat is an oral soluble guanylyl cyclase stimulator that increases cGMP production, resulting in vasodilation, improvement in endothelial function, and decreasing fibrosis. In the Vericiguat Global Study in Subjects with HFrEF (VICTORIA) trial, all-cause mortality and any-cause death or HF hospitalization were lower in the vericiguat group versus placebo.5 Owing to increased risk of hypotension, vericiguat is contraindicated with PDE5 inhibitors, riociguat, and long-acting nitrates.
Digoxin: Digoxin can be considered in patients with symptomatic HFrEF despite GDMT to decrease hospitalizations for HF.5 Studies regarding digoxin were published prior to GDMT being established. Regular monitoring should be completed to prevent digoxin toxicity from occurring.
HFpEF Medication Therapy
Treatment agents recommended for HFpEF are similar to those for HFrEF, including diuretics to improve symptoms. Until recently, clinical trials had not shown a mortality benefit or a dramatic benefit in hospitalizations in patients with HFpEF.
Treatment of HFpEF should start by optimally treating the causes of HF and controlling risk factors such as blood pressure and diabetes.5 In patients with hypertension, RAAS antagonists such as ACEis, ARBs, MRAs, and ARNis are first-line agents that can be used to treat blood pressure; however, trials of RAAS antagonists have not shown significant benefit for patients’ HFpEF.5
The recent EMPEROR-Preserved trial showed that empagliflozin had a significant benefit in time to hospitalization and in the number of HF hospitalizations.5 The study also showed improved quality-of-life scores and a slowing of eGFR decline in these patients, irrespective of diagnosis of diabetes.5
MRAs such as spironolactone have been shown to improve diastolic function and reduce hospitalization, according to the guidelines.5 The TOPCAT study also suggests a small but statistically insignificant benefit in the composite endpoint of death, aborted cardiac death, and HF hospitalization with spironolactone in patients with HFpEF.5
ARNis also have been studied in HFpEF. The PARAGON-HF study showed that compared with ARBs, ARNis did not show a significant reduction in the primary composite endpoint of cardiovascular death or HF hospitalizations in HFpEF patients. It is thought, however, that ARNIs do help reduce hospitalizations, particularly in patients with EF in the lower end of the range.5
DIFFERENCES IN DRUG THERAPIES BETWEEN SEXES
There are differences between therapies for men and women with HF. Variations in pharmacokinetics of drugs may occur owing to differences in hormones, body composition, and drug absorption, distribution, metabolism, and excretion.7,14 Women have been underrepresented in all stages of drug development, and randomized, controlled trials have not included sex-specific drug efficacy and safety evaluation.14 Women achieve the plateau of risk reduction with renin-angiotensin system inhibitors and beta-blockers at lower doses than defined target doses and do not have any additional benefit at higher doses, which poses potential discussion on the definition of optimal medical therapy for each sex.7,14 Digoxin had a higher mortality risk in women in the DIG trial. Women had higher plasma concentrations of digoxin despite being treated with lower doses of digoxin than men, resulting in more drug-related adverse effects and drug toxicity.7 In studies regarding SGLT2is, there were no sex differences regarding hospitalization rates, and benefits occurred among men and women. In the PARADIGM-HF trial, sacubitril/valsartan showed a reduction in mortality and morbidity, regardless of sex in individuals with HFrEF.14
CARDIOMYOPATHY IN PREGNANCY
Although women have a higher prevalence of HFpEF, a specific subtype of HF related to pregnancy exclusively impacts women.6
Peripartum cardiomyopathy (PPCM) is a rare idiopathic HF associated with high maternal mortality rates.15 In the U.S., cardiomyopathy is among the leading causes of pregnancy-related deaths in all age groups.16 PPCM, along with other maternal cardiovascular disease, is expected to increase and contribute to a greater number of maternal deaths worldwide in the coming years.17 Among pregnant women, 1% to 4% will have maternal cardiac disease at baseline or will develop it.16
Survival rates of women with congenital heart disease and the trend to delay motherhood until later in life continue to increase. As a result, more women tend to develop comorbidities associated with heart disease prior to pregnancy, such as diabetes, hypertension, and obesity. These trends contribute to increasing incidence of maternal heart disease and are now the most significant cause of maternal death in Western countries, even as maternal deaths from other more historically common reasons decline.17
Unfortunately, only 10% of women who die from cardiac disease during childbirth have a known heart condition prior to their event.18 The most dangerous period of pregnancy for many women with heart disease is delivery because of the potential for extreme fluid shifts, vascular resistance, and increases in cardiac output.16
Maternal mortality, HF, ventricular arrhythmias, and other adverse maternal outcomes occurred more frequently in women with cardiomyopathy than in women with other forms of heart disease.19 Women with cardiomyopathy during pregnancy were >4 times more likely to die in hospital and >7 times more likely to experience a major adverse cardiovascular event during pregnancy than women with other forms of heart disease—more specifically cardiac arrest, HF, and cardiorespiratory failure.16 Despite these alarming statistics, there is a paucity of systemic evidence to outline the potential risks of cardiomyopathy in pregnancy as well as a lack of evidence to inform optimal management strategies.16
PPCM Diagnosis
In many cases, PPCM, a subtype of cardiomyopathy, may not be recognized as such due to its symptoms overlapping with common symptoms of pregnancy, thus delaying its identification and treatment.18 In fact, its diagnosis may not be made until the postpartum period.16 PPCM is generally considered a diagnosis of exclusion, characterized by LVEF <45% with or without ventricular dilation on two-dimensional ECHO in the later months of pregnancy and a few weeks following childbirth.15
Current guideline recommendations advise against pregnancy if LVEF is <30%, although data from the worldwide Registry of Pregnancy and Cardiac disease (ROPAC) study suggest that all patients with dilated cardiomyopathy are at high risk of adverse events and should be counseled appropriately.17
The global incidence of PPCM is low (<1%), although subgroups with demonstrated higher risk of PPCM development include women of African ethnicity, specifically Nigerian heritage.15 Other factors influencing the development of PPCM include advanced maternal age, ethnicity, higher parity, multiple gestation, diabetes, family history, malnutrition, anemia, and preeclampsia.15,18,20 Notably, pregnancy-associated cardiomyopathy in childhood cancer survivors is rare, although close monitoring should be considered in patients with a history of anthracycline exposures and previous or current documented subclinical or symptomatic cardiomyopathy.21
Pathophysiology in Pregnancy and Complications
In pregnancy, the maternal cardiovascular system will undergo significant physiologic adaptations to meet the demands of the developing fetus.16 There is not a clinical consensus on the exact development of PPCM, although the “two-hit” model is the most widely accepted. In this model, it is postulated that there is vascular insult to the myocardium secondary to oxidative stress induced by prolactin-mediated cardiotoxic metabolites in genetically susceptible women.15 Other hypotheses include placental angiogenic factors causing endothelial damage and myocardial dysfunction, familial predisposition to PPCM development, and acute myocardial injury caused by immunologic mechanisms to fetal antigens.15
Studies support the theory that decompensation and limited vascular reserve play a role in higher risk for adverse outcomes in women with cardiomyopathy, as LVEF <40% predicts both immediate risk and late adverse maternal outcomes.16 Other independent predictors for adverse maternal outcome include prepregnancy HF, NYHA Class >II, modified World Health Organization Class 4, and the use of anticoagulants.17 The most common fetal risk was premature birth, occurring in 26% of individuals with cardiomyopathy who were studied.17
The results of the ROPAC study indicated that HF complicated 11% of pregnancies, 7% of those being within the first week postpartum. The authors point to these results, which highlights the need for intensive management and monitoring of women at risk for HF before conception, throughout pregnancy, and after delivery.17
PPCM Treatment and Management
For family planning, it is important that patients who are at high risk of PPCM receive preconception counseling by an obstetrician, obstetric physician, or cardiologist with special interest in maternal cardiology and that cardiac function is optimized prior to pregnancy.17,18
An important and easily treatable risk factor for PPCM is anemia.15 Other modifiable risk factors for PPCM include asthma, smoking, thyroid disease, and preeclampsia.15,20 Thyroid hormone supplementation, adequate asthma and blood pressure management, and smoking cessation are reasonable to consider for improving maternal and fetal outcomes.20
Most studies emphasize delivery at the earliest safe gestational point as the gold standard of PPCM management.15 It is widely accepted that vaginal delivery is preferred over cesarean section as a means of delivery owing to lower blood loss and reduced risk of anesthetic or surgery-related complications.15 Prior to delivery, medical therapy with all appropriate pillars of HF treatment—weighing maternal and fetal risk—remains the standard of care.15 ACEis/ARBs are not recommended in the first trimester owing to higher risk of fetal malformation, and they are contraindicated in the second and third trimesters.18
PPCM Postpartum and Lactation
Approximately 50% to 80% of women will make a full recovery from PPCM within 3 to 6 months and may eventually be able to wean off medical therapy after 12 months; however, there is significant risk of developing PPCM in a subsequent pregnancy.15,18 Recovery from PPCM is defined as LVEF >55%.22
Many patients are counseled to stop or avoid breastfeeding at the time of their PPCM diagnosis owing to concerns about prolactin’s role in driving PPCM pathogenesis. 22 Bromocriptine is being considered as a therapy option to suppress prolactin and is being studied in the ongoing Randomized Evaluation of Bromocriptine in Myocardial Recovery Therapy (REBIRTH) study.23
Several expert academies maintain that multiple HF medications, including metoprolol, carvedilol, captopril, enalapril, benazepril, digoxin, and spironolactone, can be safely used during lactation.22 Lactation safety data remain insufficient for ARNi and SGLT2is.22
PHARMACIST’S ROLE
Pharmacists play a crucial role in the management of HF. Roles and responsibilities include medication reconciliation, identifying and resolving drug-related concerns, and promoting medication adherence. Depending on the setting, pharmacists have the opportunity to optimize GDMT to help patients achieve target doses of medications.24 Pharmacists can provide education to patients and caregivers regarding disease state and treatment management. As new pharmacologic treatments become available, pharmacists can provide education regarding efficacy and safety. Pharmacists are an integral part of the multidisciplinary team in managing HF.25
CONCLUSION
HF is a growing cause of morbidity and mortality in women, and it is important to expand scientific knowledge about the nuances of physiology, treatment, and outcomes between women and men. It is important to include more women in research so that guidelines and recommendations can optimize treatment for all. Moving forward, studies must unravel sex-specific mechanisms— such as the higher prevalence of HFpEF and the unique challenge of PPCM—to guide precise GDMT that accounts for hormonal and pharmacokinetic differences. Enhanced female enrollment in trials, integration of pharmacist-led medication optimization, and collaboration between cardiology and obstetric specialists will ensure equitable, tailored prevention and management strategies that improve quality of life and survival for women with HF.
REFERENCES
- Martin SS, Aday AW, Allen NB, et al. 2025 heart disease and stroke statistics: a report of US and global data from the American Heart Association. Circulation. 2025;151(8):e41-e660.
- Bozkurt B, Ahmad T, Alexander K, et al. HF STATS 2024: heart failure epidemiology and outcomes statistics an updated 2024 report from the Heart Failure Society of America. J Card Fail. 2025;31(1):66- 116.
- DeFilippis EM, Beale A, Martyn T, et al. Heart failure subtypes and cardiomyopathies in women. Circ Res. 2022;130(4):436-454.
- Khan SS, Beach LB, Yancy CW. Sex-based differences in heart failure: JACC Focus Seminar 7/7. J Am Coll Cardiol. 2022;79(15):1530- 1541.
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/ HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2022;145(18):e895-e1032.
- Lala A, Tayal U, Hamo CE, et al. Sex differences in heart failure. J Card Fail. 2022;28(3):477-498.
- Rosano GMC, Stolfo D, Anderson L, et al. Differences in presentation, diagnosis and management of heart failure in women. A scientific statement of the Heart Failure Association of the ESC. Eur J Heart Fail. 2024;26(8):1669-1686.
- Bozkurt B, Khalaf S. Heart failure in women. Methodist Debakey Cardiovasc J. 2017;13(4):216-223.
- Kenchaiah S, Vasan RS. Heart failure in women—insights from the Framingham Heart Study. Cardiovasc Drugs Ther. 2015;29(4):377- 390.
- Shah SP, Dixit NM, Mendoza K, et al. Integration of clinical pharmacists into a heart failure clinic within a safety-net hospital. J Am Pharm Assoc. 2022;62:575-579.e2.
- McMurray JJV, Packer M. How should we sequence the treatments for heart failure and a reduced ejection fraction? A redefinition of evidence-based medicine. Circulation. 2021;143:875-877.
- Tromp J, Ouwerkerk W, van Veldhuisen DJ, et al. A systematic review and network meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2022;10:73-84.
- Mohebi R, Liu Y, Piña I, et al. Dose-response to sacubitril/ valsartan in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2022;80:1529-1541.
- Arata A, Ricci F, Khanji MY, et al. Sex differences in heart failure: what do we know? J Cardiovasc Dev Dis. 2023;10(7):277.
- Metgud SC, Patil A, Renuka PM. Epidemiology, risk factors, and pregnancy outcomes in peripartum cardiomyopathy. J Sci Soc. 2025;52:43-48.
- Eggleton EJ, McMurrugh KJ, Aiken CE. Maternal pregnancy outcomes in women with cardiomyopathy: a systematic review and meta-analysis. Am J Obstet Gynecol. 2022;227(4):582-592.
- Roos-Hesselink J, Baris L, Johnson M, et al. Pregnancy outcomes in women with cardiovascular disease: evolving trends over 10 years in the ESC Registry Of Pregnancy And Cardiac disease (ROPAC). Eur Heart J. 2019;40(47):3848-3855.
- Jarman R. Peripartum cardiomyopathy. AIMS J. 2024;36(2):1- 7.
- van der Zande JA, Greutmann M, Tobler D, et al. Diuretics in pregnancy: data from the ESC Registry of Pregnancy and Cardiac disease (ROPAC). Eur J Heart Fail. 2024;26(7):1561-1570.
- Afana M, Brinjikji W, Kao D, et al. Characteristics and inhospital outcomes of peripartum cardiomyopathy diagnosed during delivery in the United States from the Nationwide Inpatient Sample (NIS) database. J Card Fail. 2016;22(7):512-519.
- Hines M, Mulrooney D, Hudson M, et al. Pregnancy-associated cardiomyopathy in survivors of childhood cancer. J Cancer Surviv. 2016;10(1):113-121.
- Noll A, Kawamoto KR, Dassanayake MT, et al. Breastfeeding in patients with peripartum cardiomyopathy: clinical outcomes and physician counseling. Int Breastfeed J. 2024;19(73):1-4.
- National Heart, Lung, and Blood Institute. Impact of Bromocriptine on Clinical Outcomes for Peripartum Cardiomyopathy (REBIRTH). Dennis M. McNamara, MD, MS; 2025. https://research.ebsco.com/ linkprocessor/plink?id=4f70e8d9-0179-33f6-ac7f-b1ac35c787be. Accessed June 30, 2025.
- Twydell B, Kelsh S, Dickinson M, et al. Pharmacist-led guidelinedirected medical therapy (GDMT) clinic for the optimization of medications for heart failure with reduced ejection fraction. J Card Fail. 2025;31(1):228.
- Anderson SL, Marrs JC. A review of the role of the pharmacist in heart failure transition of care. Adv Ther. 2018;35(3):311-323.
- Masarone D, Martucci ML, Errigo V, Pacileo G. The use of β-blockers in heart failure with reduced ejection fraction. J Cardiovasc Dev Dis. 2021;8(9):101.